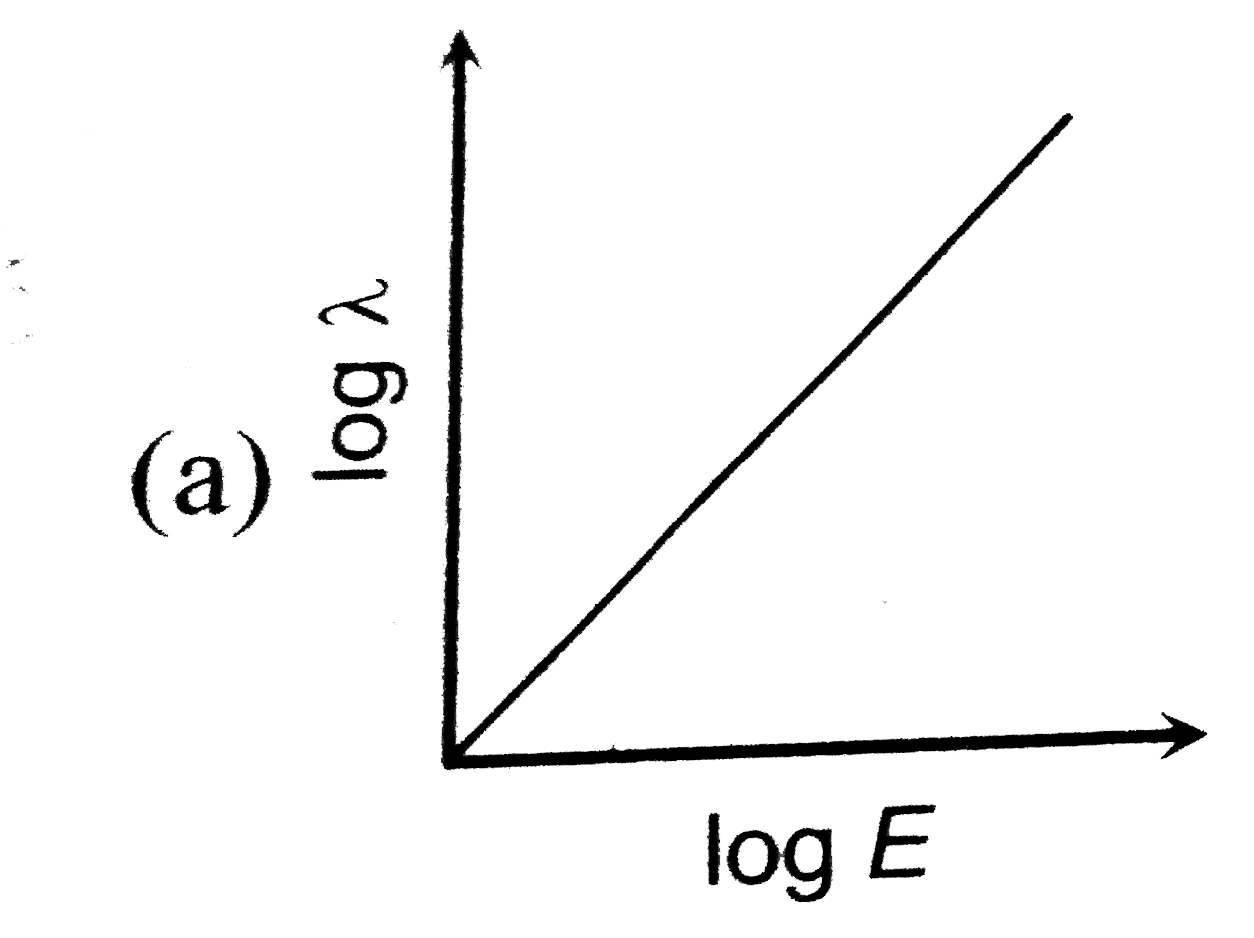
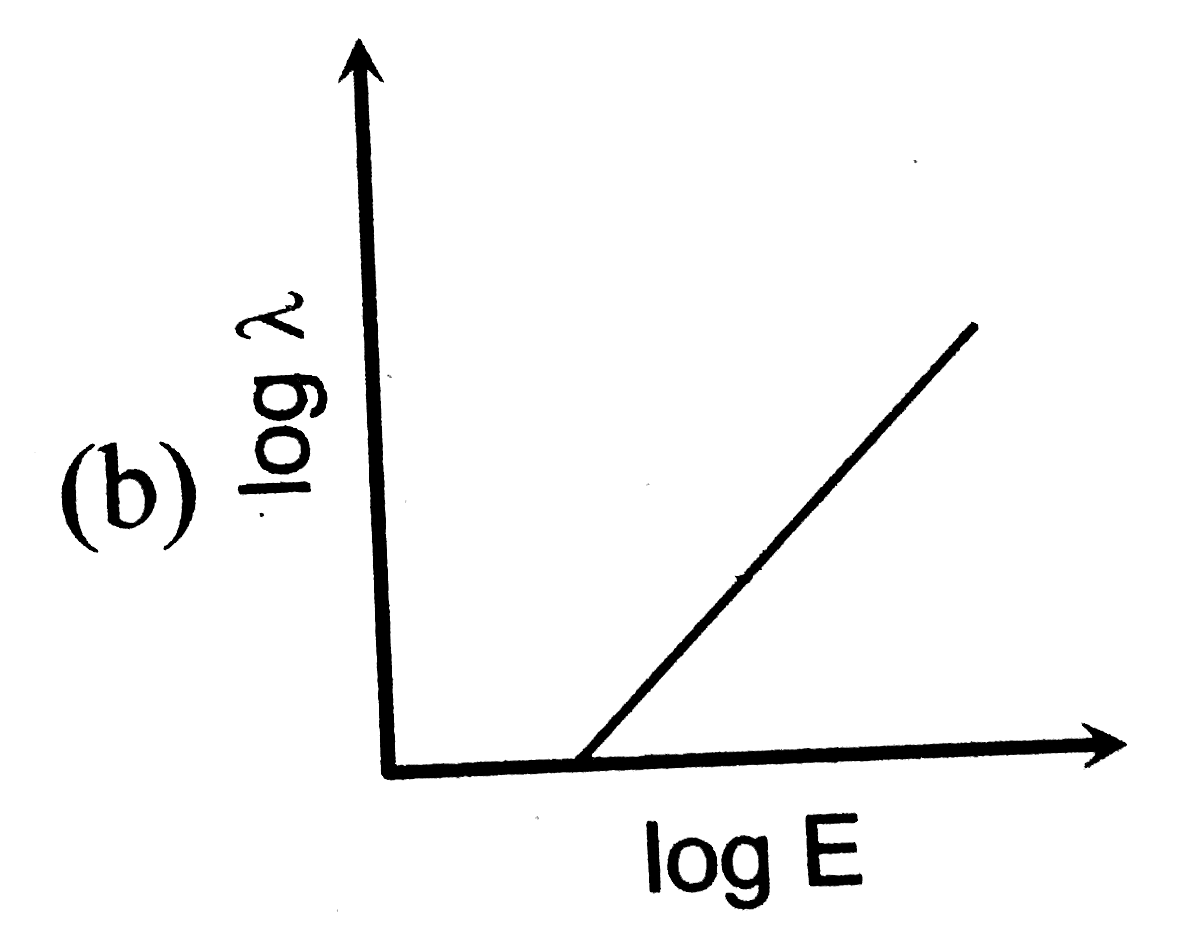
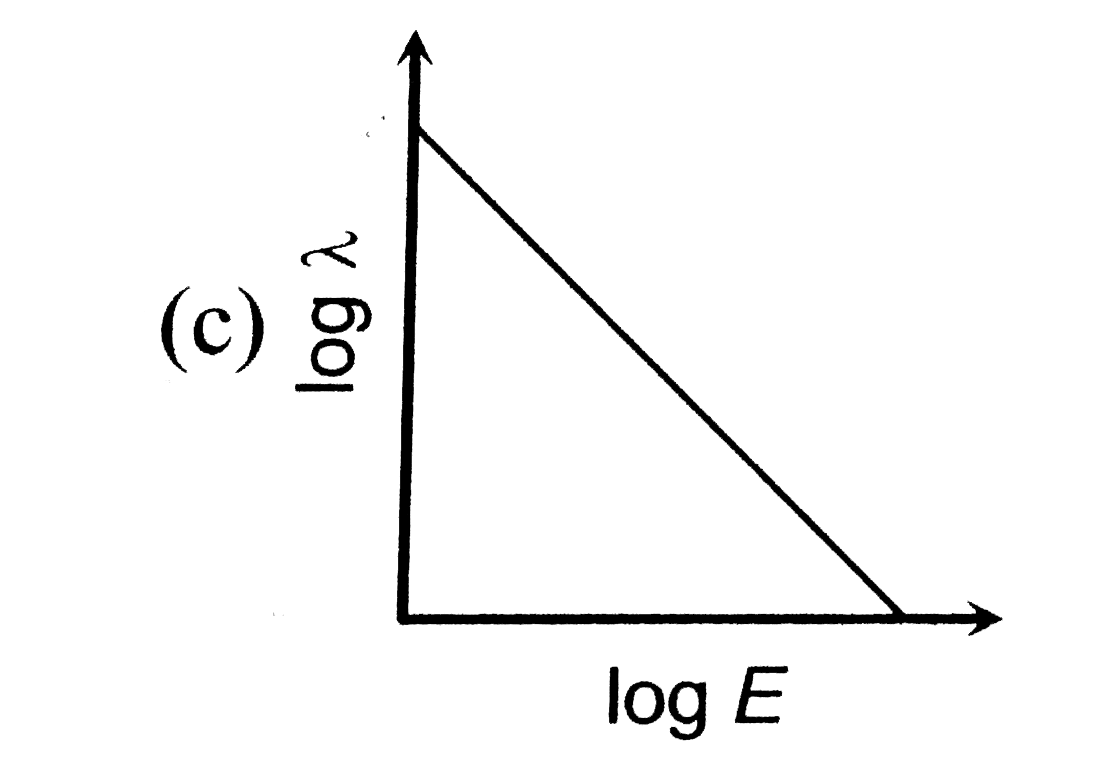
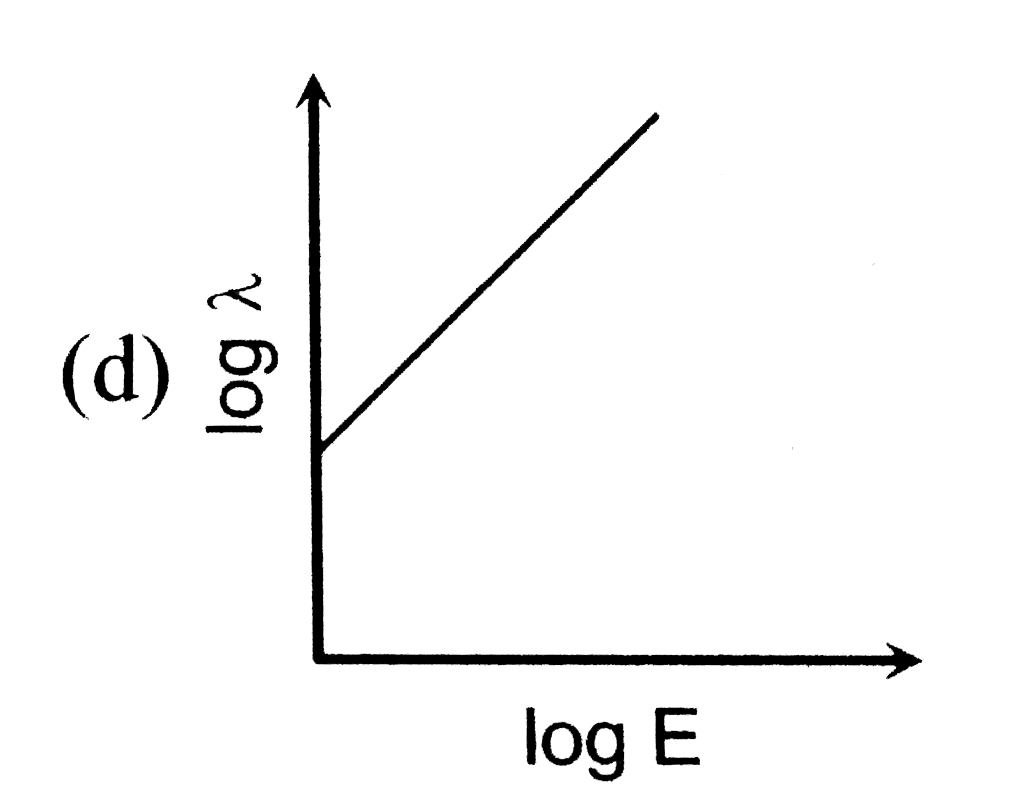
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
DUAL NATURE OF RADIATION AND MATTER
A2Z|Exercise Photo Momentum Energy|30 VideosDUAL NATURE OF RADIATION AND MATTER
A2Z|Exercise Photo Electric Effect|61 VideosCURRENT ELECTRICITY
A2Z|Exercise Section D - Chapter End Test|29 VideosELECTRIC CHARGE, FIELD & FLUX
A2Z|Exercise Section D - Chapter End Test|29 Videos
Similar Questions
Explore conceptually related problems
A2Z-DUAL NATURE OF RADIATION AND MATTER-Section D - Chapter End Test
- The log - log graph between the energy E of an electron and its de - B...
Text Solution
|
- If a photon has velocity c and frequency n , then which of following r...
Text Solution
|
- Lights of two different frequencies whose photons have energies 1 and ...
Text Solution
|
- Sodium and copper have work functions 2.3 eV and 4.5 eV respectively ....
Text Solution
|
- Two identical photocathodes receive light of frequency f(1) andf(2) if...
Text Solution
|
- The work function of a substance is 4.0 eV. The longest wavelength of ...
Text Solution
|
- According to Einstein's photoelectric equation, the plot of the maximu...
Text Solution
|
- A photocell is illuminated by a small bright source places 1 m away w...
Text Solution
|
- If the kinetic energy of a free electron doubles , its de - Broglie wa...
Text Solution
|
- In a photoelectric effect , the K.E. of electrons emitted from the met...
Text Solution
|
- The photoelectric effect can be understood on the basis of
Text Solution
|
- If the threshold wavelength for sodium is 5420 Å, then the work functi...
Text Solution
|
- The magnitude of saturation photoelectric current depends upon
Text Solution
|
- For photoelectric emission , tungsten requires light of 2300 Å. If lig...
Text Solution
|
- The light rays having photons of energy 1.8 eV are falling on a metal ...
Text Solution
|
- A photon of energy 8 eV is incident on metal surface of threshold fre...
Text Solution
|
- In the diagram a graph between the intensity of X-rays emitted by a mo...
Text Solution
|
- The maximum value of stopping potential in the following diagram is
Text Solution
|
- The variation of wavelength lambda of the K(alpha) line with atomic nu...
Text Solution
|
- From the figure describing photoelectric effect we may infer correctly...
Text Solution
|
- When an inert gas is filled in the place vacuum in a photo cell , then
Text Solution
|