A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
A2Z|Exercise Entropy, Gibb'S Energy And Spontaneity Of Process|52 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Heat Capacity And Calorimetry|13 VideosCHEMICAL THERMODYNAMICS
A2Z|Exercise Section D - Chapter End Test|30 VideosCHEMICAL EQUILIBRIUM
A2Z|Exercise Section D - Chapter End Test|30 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY OF PROPERTIES
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-CHEMICAL THERMODYNAMICS-Work, Internal Energy And Enthalpy
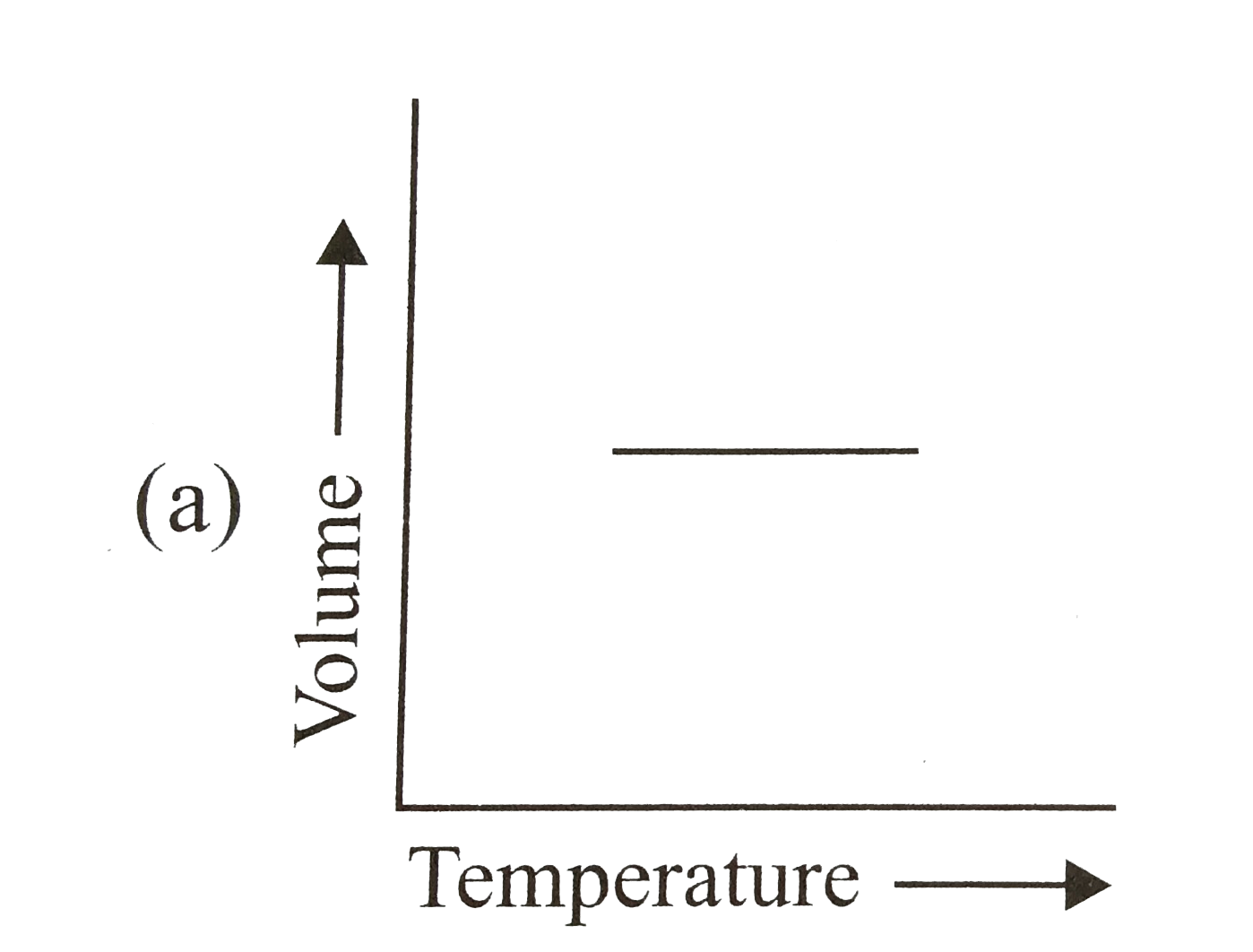
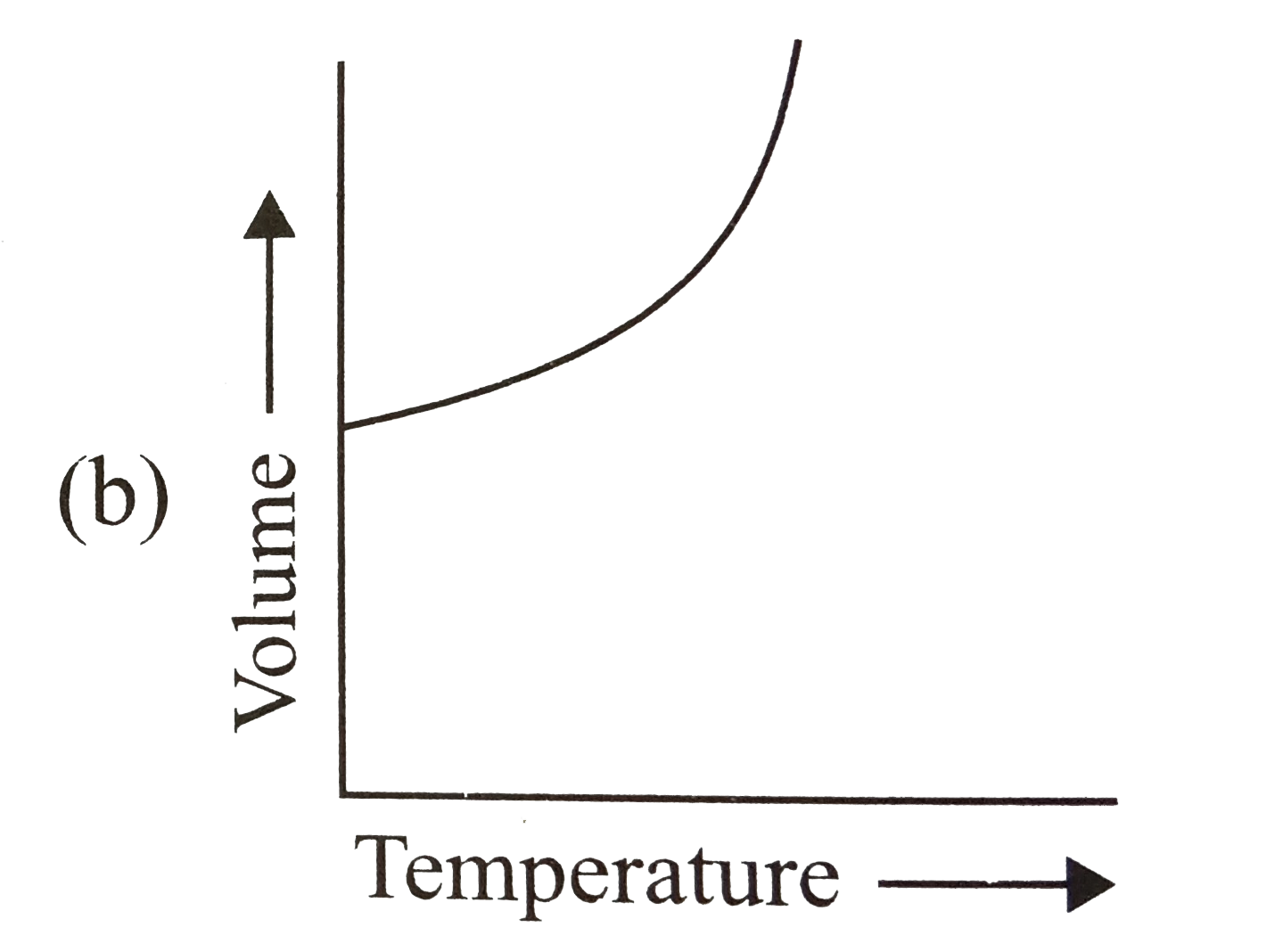
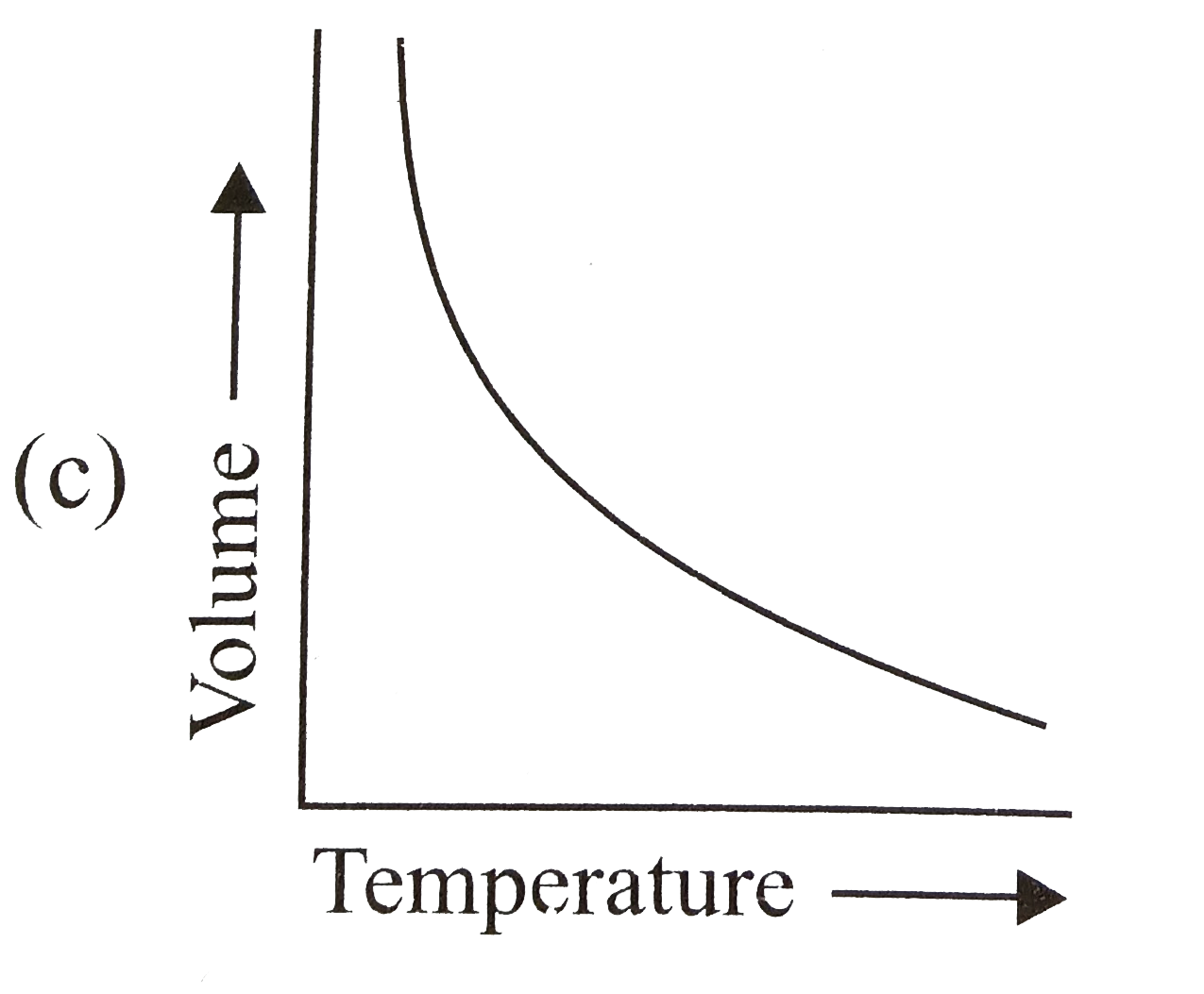
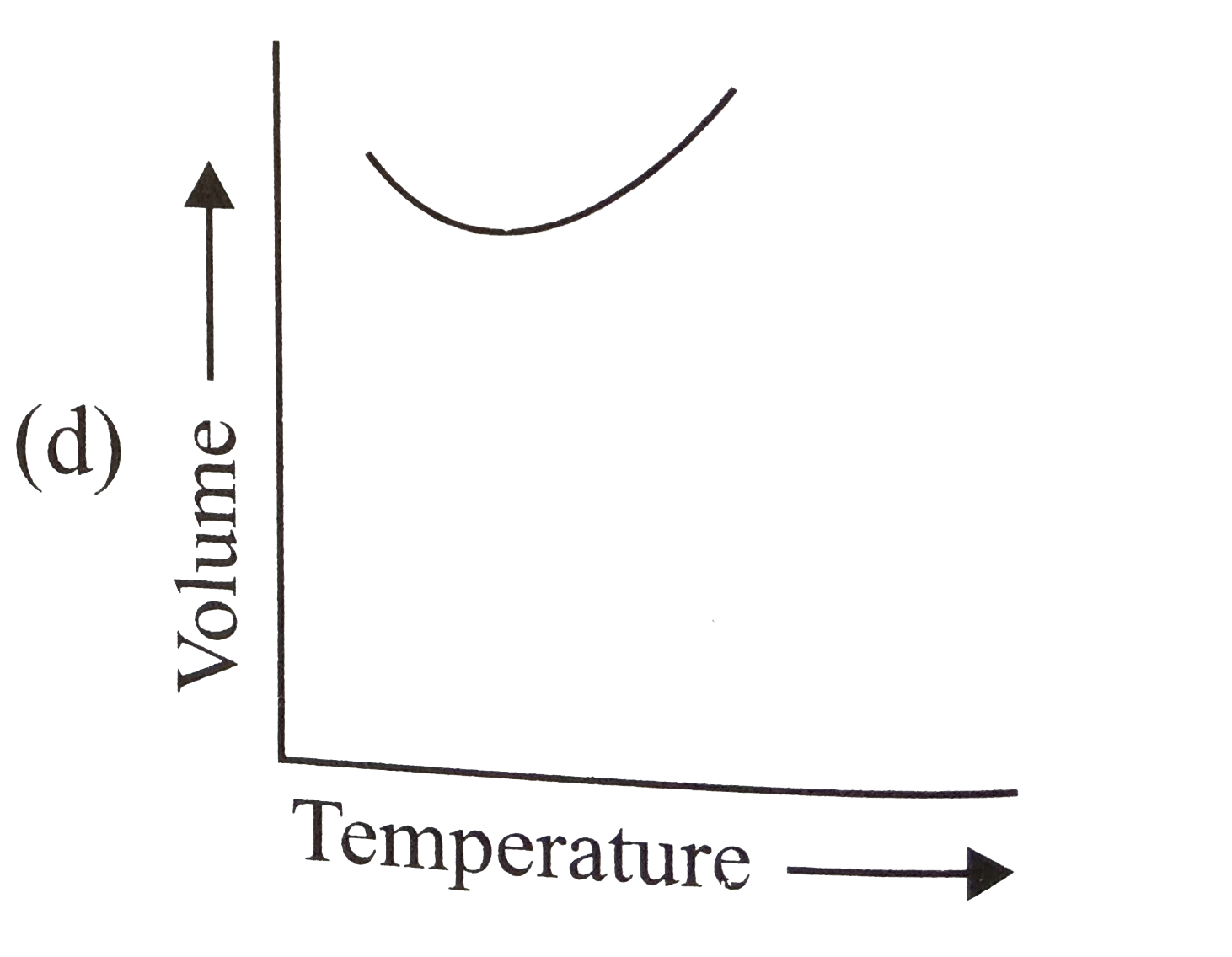
- The relation between the volume and temperature of a sample of water i...
Text Solution
|
- One gram-atom of graohite and one gram-atom of diamond were separately...
Text Solution
|
- For the raction B(2)H(6)(g)+3O(2)(g)rarrB(2)O(2)(s)+3H(2)O(l) Delt...
Text Solution
|
- For the reaction, X(2)O(4)(l)rarr2XO(2)(g),DeltaE=2.1Kcal , DeltaS...
Text Solution
|
- Combustion of methance
Text Solution
|
- 10mol of an ideal gas confined to a volume of 10L is released into atm...
Text Solution
|
- NH(3)(g)+3C1(2)(g)rarrNCL(3)(g)+3HC1(g)DeltaH(1) N(2)(g)+3H(2)(g)rar...
Text Solution
|
- The enthalpy change for a reaction does not depend upon:
Text Solution
|
- Enthalpy of combustion of CH(4) , C(2)H(6) and C(3)H(8) are -210-370 a...
Text Solution
|
- Given, H(2)(2)+Br(2)(g)rarr2HBr(g) , Deltah(1)^(@) and standerd enthal...
Text Solution
|
- The heat of combustion of yellow phoshphorus and red phosphorus are -9...
Text Solution
|
- The enthalpies of combustion of carbon and carbon monoxide are -393.5 ...
Text Solution
|
- The heat of formation of C(2)H(5)OH(l) is -66Kcal//mol . The heat of c...
Text Solution
|
- Given that C+O(2)rarrCO(2),DeltaH^(@)=-xKJ and 2CO+O(2)rarr2CO(2),Delt...
Text Solution
|
- Assuming that water vapour is an ideal gas, the internal energy change...
Text Solution
|
- Given that C+2SrarrCS(2)DeltaH^(@)f=+117.0KJmol^(-1) (1) C+O(2)rarrC...
Text Solution
|
- The enthaply at 298K of the reaction H(2)O(2)(l)rarr+(1)/(2)O(2)(g) ...
Text Solution
|
- Equal volumes of molar hydrochloric acid and subphuric acid are neutra...
Text Solution
|
- From the standard enthalpies of formation values for some compounds (i...
Text Solution
|
- Delta H(1)^(@) for CO(2)(g), CO(g) and H(2)O(g) are -393.5,-110.5 and ...
Text Solution
|