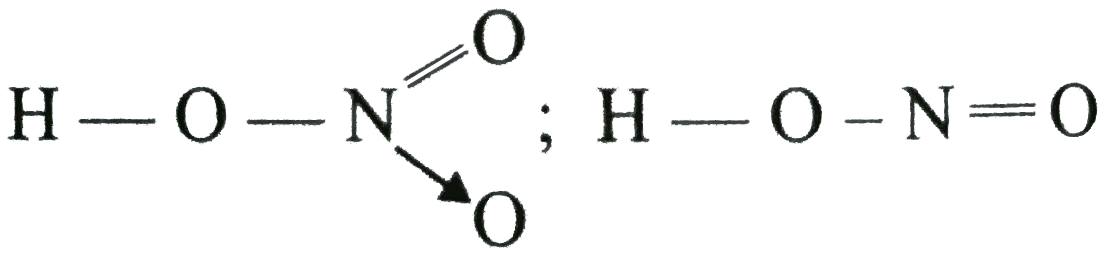A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
P BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Exercise|7 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise Group 17 (Halogen Family)|22 VideosP BLOCK ELEMENTS ( GROUP 15,16,17,18)
A2Z|Exercise AIIMS Questions|25 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
A2Z|Exercise Section D - Chapter End Test|30 VideosSOLID STATE
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-P BLOCK ELEMENTS ( GROUP 15,16,17,18)-Assertion-Reasoning Questions
- HNO3 is a stronger acid than HNO2 In HNO2, there are two nitrogen to...
Text Solution
|
- Asseration: Ammonia and water are electron rich hydrides. Reason: T...
Text Solution
|
- Asserarion: Phosphine is prepared in an inert atmoshphere of CO(2) and...
Text Solution
|
- Asseration: Both H(3)PO(4) and H(3)PO(3) posses the same number of hyd...
Text Solution
|
- Asseration: White phosphorus is less stable whereas red phosphorus is ...
Text Solution
|
- Asseration: H(3)PO(4) and fH(3)PO(3) both are present in fertilizers. ...
Text Solution
|
- Oxygen molecule exhibits
Text Solution
|
- Copper molecule exhibits
Text Solution
|
- Which of the following is acidic?
Text Solution
|
- Oxalic acid when heated with conc. H2 SO4 it gives out
Text Solution
|
- H(2)S reacts with O(2) to form?
Text Solution
|
- Shape of O2F2 is similar to that of
Text Solution
|
- Bleaching action of SO(2) is due to :
Text Solution
|
- Asseration: SeCl(4), does not havea tetrahedral structure. Reason: S...
Text Solution
|