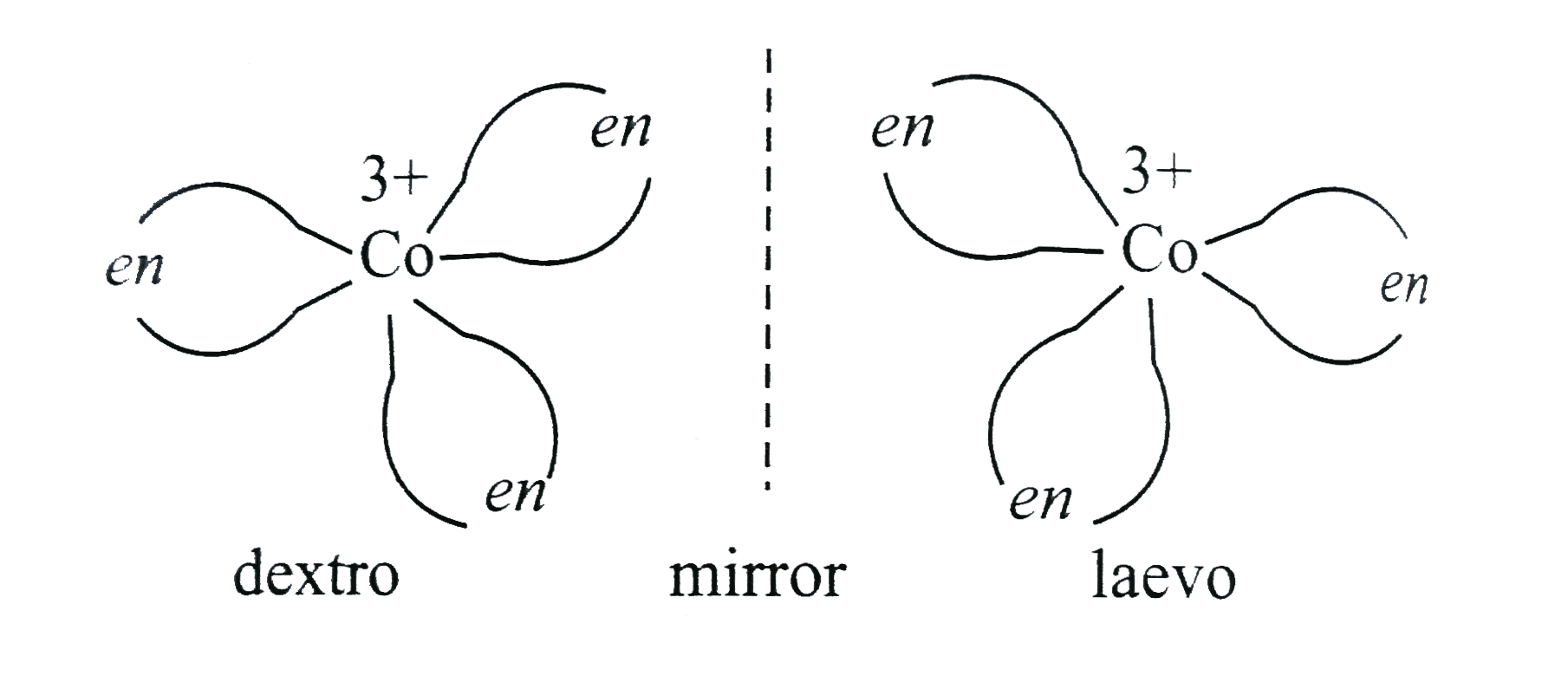
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
A2Z|Exercise Wemer Theory And Vbt|42 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Crystal Field Theory (Cft)|16 VideosCOORDINATION COMPOUNDS
A2Z|Exercise Structural Isomerism|31 VideosCHEMICAL KINETICS
A2Z|Exercise Section D - Chapter End Test|30 VideosELECTROCHEMISTRY
A2Z|Exercise Section D - Chapter End Test|30 Videos
Similar Questions
Explore conceptually related problems
A2Z-COORDINATION COMPOUNDS-Optical Isomerism
- Type of isomerism which is shown by [PtCl2Br2]^(2-) is also observed i...
Text Solution
|
- Which of the following statements is not true about the complex ion [P...
Text Solution
|
- Tris (ethylenediamine) cobalt (III) cation, [Co(en)3]^(3+), can have
Text Solution
|
- Which one of the following complexes exhibit chirality?
Text Solution
|
- Which of the following complex will show optical activity?
Text Solution
|
- Which of the following is considered to be an anticancer species ?
Text Solution
|
- [Co(en)3]^(3+) ion is expected to show
Text Solution
|
- In which case racemic mixture is obtained on mixing its mirror images ...
Text Solution
|
- The complex that exists as a pair of enantiomers is
Text Solution
|
- Which of the following complexes exhibit optical isomerism?
Text Solution
|
- The complex shown below can exhibit
Text Solution
|
- Which of the following has the largest number of isomers? .
Text Solution
|
- Which of the following will show optical isomerism? .
Text Solution
|
- Which of the folloiwng complexes in not expected to exhibit optical is...
Text Solution
|
- Which of the following has an optical isomer? (en=ethylenediamine) ?
Text Solution
|
- Which of the following has an optical isomer?
Text Solution
|
- The number of isomeric forms in which [Co(NH3)4Cl2]^(+) ion can occur ...
Text Solution
|
- The phenomenon of optical activity will be shown by:
Text Solution
|
- Which of the following compound shows optical isomerism? (en=ethylened...
Text Solution
|
- When [Ni(NH(3))(4)]^(2+) is treated with conc HCI, two compounds havin...
Text Solution
|