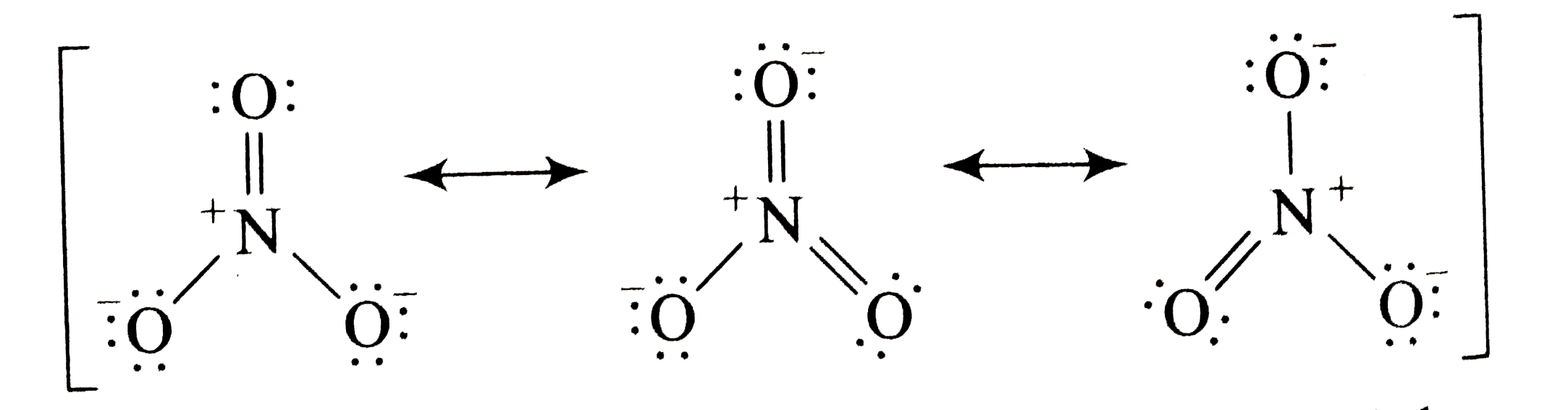
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 7|12 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 8|10 VideosCHEMICAL BONDING
R SHARMA|Exercise Follow-up Test 5|7 VideosAROMATIC HYDROCARBONS
R SHARMA|Exercise Archives|59 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-CHEMICAL BONDING-Follow-up Test 6
- Which of the following is incorrect regarding resonance?
Text Solution
|
- A molecule is described by three Lewis structures having energies E1, ...
Text Solution
|
- How many resonance structures can be drawn for the nitrate ion, NO3^(-...
Text Solution
|
- Which of the following ions has resonating structures?
Text Solution
|
- Which of the following can exhibit resonance? (i) Oxygen (ii) Ozone ...
Text Solution
|
- Which of the following resonating structures is not correct for CO2? ...
Text Solution
|
- How many resonating structures can be drawn for NO2?
Text Solution
|