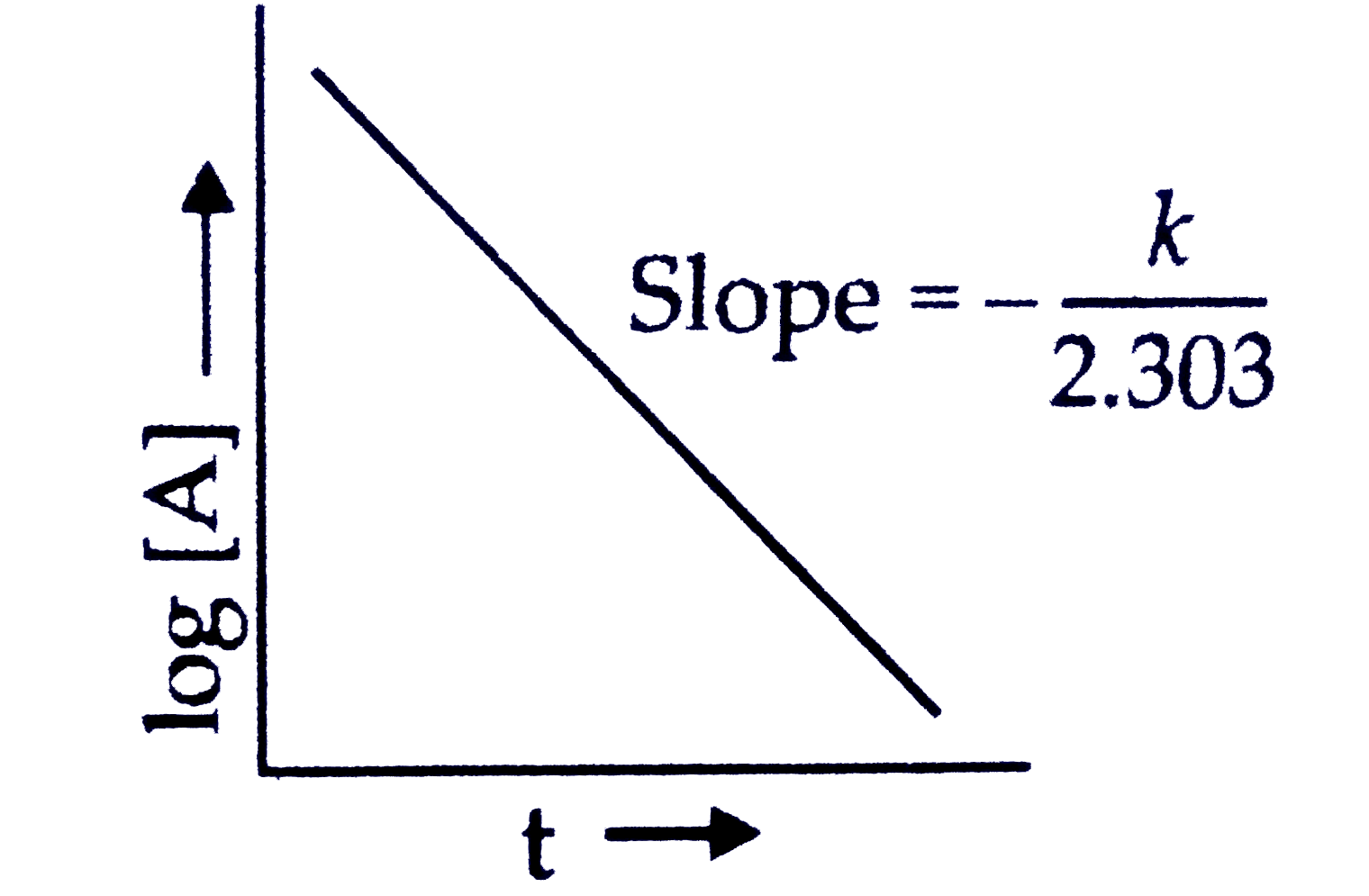
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NIKITA PUBLICATION-CHEMICAL KINETICS -MCQs
- The reaction , X + 2Y + Z rarr N occurs by the following mechanism (...
Text Solution
|
- When a plot of log k versus 1//T of chemical reaction is made, the ene...
Text Solution
|
- For a first order reaction, the reaction, the plot of log C against 't...
Text Solution
|
- For a general chemical change 2A+3B rarr Products, the rates with res...
Text Solution
|
- A reaction is represented by A overset(k1)rarr B (slow ) and A+B over...
Text Solution
|
- In several experiments on the kinetics of reaction A+B to Products, it...
Text Solution
|
- For the reaction, A+2B +C to D +2E, the rate of formation of D is foun...
Text Solution
|
- The hydrolysis of methyl formate in acid solution has rate expression,...
Text Solution
|
- For an endothermic reaction, where Delta H represents the enthalpy of ...
Text Solution
|
- Th Arrhenius equation expressing the effect of temperature on the rate...
Text Solution
|
- The minimum amount of energy required for the reacting molecules to un...
Text Solution
|
- If E(f) and E(r) are the activation energies of forward and backward r...
Text Solution
|
- Two reactions A to products and B to products have rate constants KA a...
Text Solution
|
- Combustion of carbon is exothermic, but coal stored in coal depots doe...
Text Solution
|
- Increase in the concentration of the reactants leads to the changes in
Text Solution
|
- According to collision theory of reaction rates
Text Solution
|
- Which statement is not correct?
Text Solution
|
- The Activation energy for a chemical reaction mainly depends upon
Text Solution
|
- For an exothermic reaqction, the energy of activation of the reactants...
Text Solution
|
- The rate of reaction increases with increase of temperature because a
Text Solution
|