A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALDEHYDES AND KETONES
ERRORLESS |Exercise Ordinary Thinking (Objective Questions) Preparation|43 VideosALDEHYDES AND KETONES
ERRORLESS |Exercise Ordinary Thinking (Objective Questions) Properties|207 VideosALCOHOL, PHENOL AND ETHER
ERRORLESS |Exercise JEE SECTION (JEE Advanced 2018) More than one choice correct answer|1 VideosANALYTICAL CHEMISTRY
ERRORLESS |Exercise JEE Section (Only One Choice Answer)|36 Videos
Similar Questions
Explore conceptually related problems
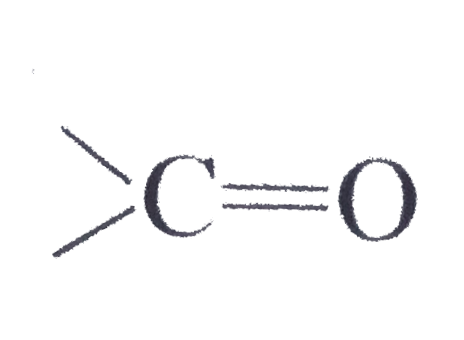 group?
group?