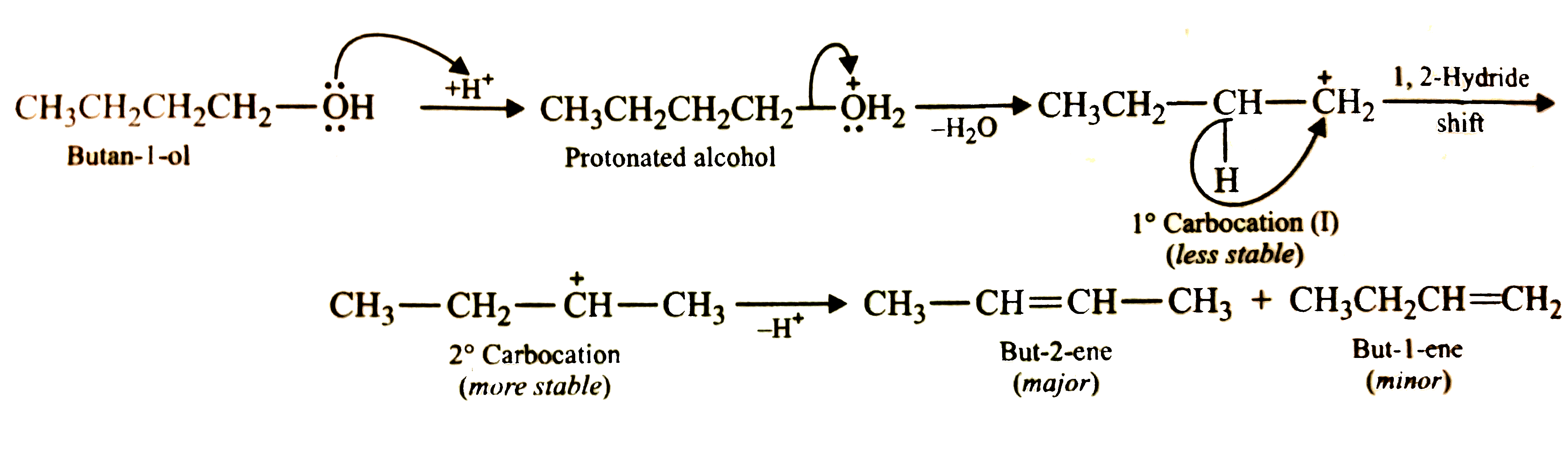Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT EXERCISES)|33 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (MULTIPLE CHOICE QUESTIONS-I)|16 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT SOLVED QUESTIONS)|7 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALCOHOLS, PHENOLS AND ETHERS-NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS)
- Classify the following as primary, secondary and tertiary alcohols (...
Text Solution
|
- Choose an allylic alcohol form the given options.
Text Solution
|
- Name the following compounds according to the IUPAC system: (i) CH(3...
Text Solution
|
- Show how are the following alcohols prepared by the reaction of a suit...
Text Solution
|
- Write structures of the products of the following reactions.
Text Solution
|
- Give structures of the products you would expect when each of the foll...
Text Solution
|
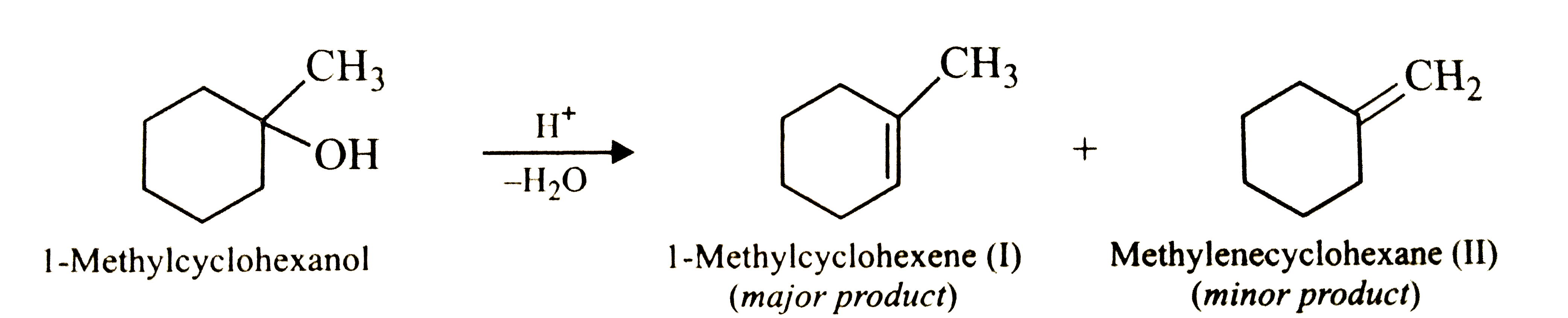
- Predict the major product of acid catalysed dehydration of (i) 1-met...
Text Solution
|
- Ortho and para nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- Write the equations involved in the following reactions: (i) Reimer ...
Text Solution
|
- Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentan...
Text Solution
|
- Which of the following is an appropriate set of reactants for the prep...
Text Solution
|
- Predict the products of the following reactions: (i) CH(3)-CH(2)-CH(...
Text Solution
|