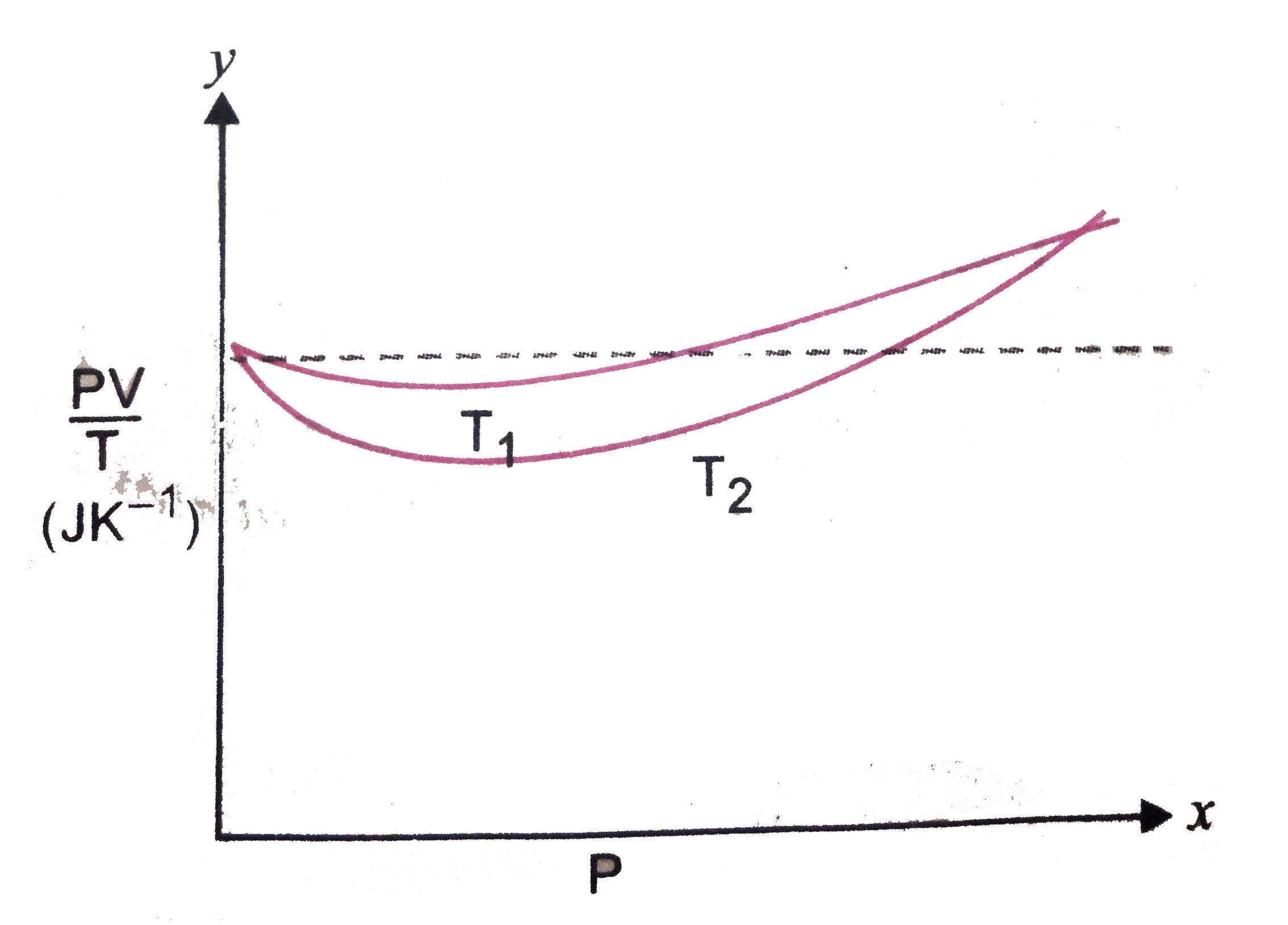Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-KINETIC THEORY OF GASES-SOLVED EXAMPLE
- Fig shows of PV//T versus P for 1.00 xx 10^(-3) kg of oxygen gas at tw...
Text Solution
|
- A vessel contains two non-reactive gases neon (monoatomic) and oxygen ...
Text Solution
|
- The total number of air molecules in a room of capacity 20 m^(3) at a ...
Text Solution
|
- A vessel is filled with a gas at a pressure of 76 cm of mercury at a c...
Text Solution
|
- What is the total kinetic energy of 2g of Nitrogen gas at temperature ...
Text Solution
|
- Your are given the following group of particles, n(i) represents the n...
Text Solution
|
- Two vessels have equal volums. One of them contains hydrogen at one at...
Text Solution
|
- At what temperature , will the rms speed of oxygen molecules be suffic...
Text Solution
|
- Calculate (i) rms velocity and (ii) mean kinetic energy of one gram mo...
Text Solution
|
- Two moles of an idel gas X occupying a volume V exerts a pressure P. T...
Text Solution
|
- Three vessel of equal capacity have gases at the same temperature and ...
Text Solution
|
- At what temperature is the root mean square speed of an atom in an arg...
Text Solution
|
- A nitrogen molecule at the surface of earth happens to have 'rms' spee...
Text Solution
|
- A gas mixture consists of 2 moles of oxygen and 4 moles of argon at te...
Text Solution
|
- One kg of a diatomic gas is at pressure of 8xx10^4N//m^2. The density ...
Text Solution
|
- Calculate the total kinetic energy of one kilo mole of Oxygen gas at 2...
Text Solution
|
- Two perfect gases at absolute temperature T(1) and T(2) are mixed. The...
Text Solution
|
- A cylinder of fixed capacity 22.4 litre contains helium gas at standar...
Text Solution
|
- A gas has molar heat capacity C = 37.55 J "mole"^(-1)K^(-1), in the pr...
Text Solution
|
- Estimate the mean free path for a water molecule in water vapour at 37...
Text Solution
|