A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-D BLOCK ELEMENTS-EXERCISE-3 PART-II
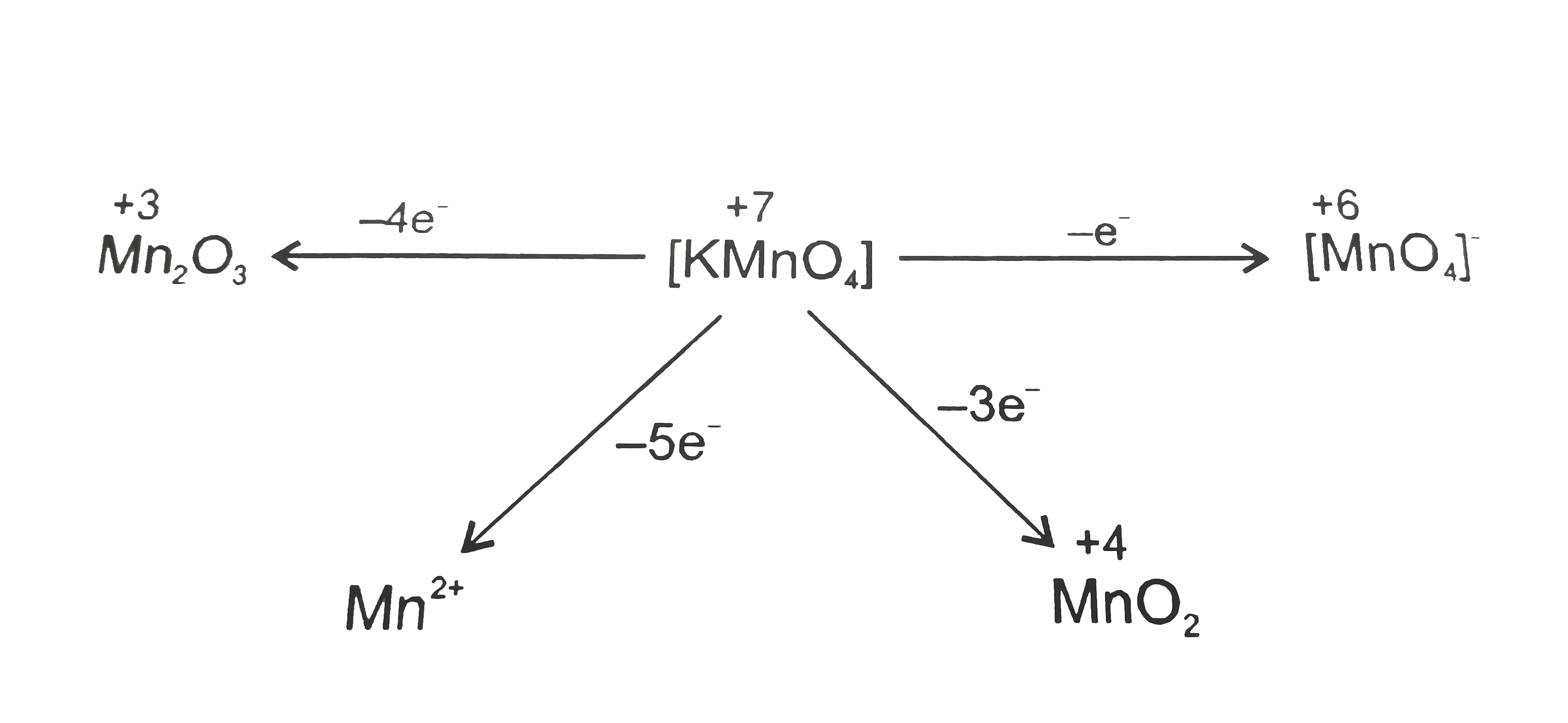
- Number of electrons transferred in each case when KMnO(4) acts as an o...
Text Solution
|
- Which of the following ion has the maximum magnetic moment?
Text Solution
|
- The most common oxidation states of cerium are
Text Solution
|
- What happen when a solution of potassium chromate is treated with an e...
Text Solution
|
- Which one of the following nitrates will leaves behind a metal on stro...
Text Solution
|
- The atomic number of V,Cr, Mn and Fe are respectively 23,24,25 and 26....
Text Solution
|
- Which of the following group of transition metals is called coinage me...
Text Solution
|
- The number of d-electrons retained in Fe^(2+) (At. No. Fe=26) ions are...
Text Solution
|
- Ammonia form the complex ion[Cu(NH(3))(4)]^(2+) with copper ions in t...
Text Solution
|
- The radius of La^(3+)(Z=57) is 106 pm. Which one of the following give...
Text Solution
|
- Cerium (Z= 58) is an important nember of the lanthanoids . Which of th...
Text Solution
|
- The lanthanide contraction is responsible for the fact that
Text Solution
|
- Which of the following factors may be regarded as the main cause of la...
Text Solution
|
- The spin-only magnetic moment [in units of Bohr magneton, (mu(B) of Ni...
Text Solution
|
- Lanthanoid contraction is caused due to:
Text Solution
|
- Identify the incorrect statement among the following.
Text Solution
|
- The actinoids exhibit more number of oxidation states in general than ...
Text Solution
|
- Larger number of oxidation state are exhibited by the actinoids than t...
Text Solution
|
- In context with the transition element, which of the following stateme...
Text Solution
|
- Knowing that the chemistry of lanthanoids (Ln) is dominated by its +3 ...
Text Solution
|