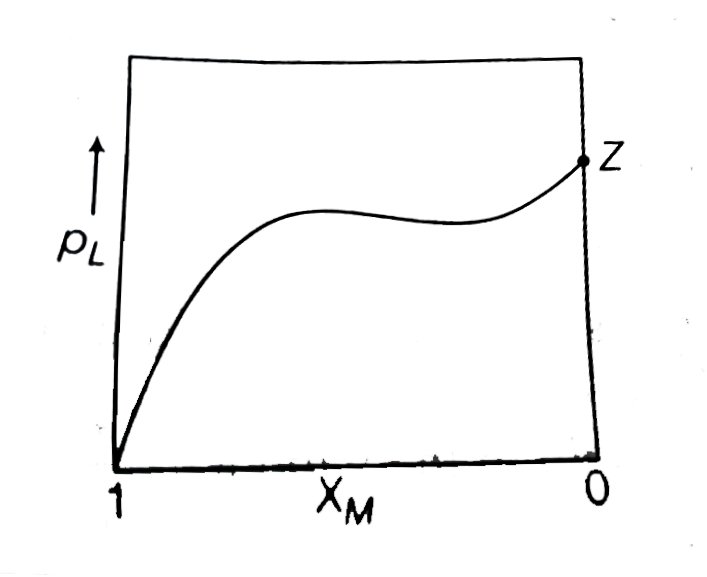
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS AND COLLIGATIVE PROPERTIES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise TOPIC 2 (Objective Questions I)|1 VideosSOLUTIONS AND COLLIGATIVE PROPERTIES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Test|4 VideosSOLUTIONS AND COLLIGATIVE PROPERTIES
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Assertion-Reason Type|2 VideosSOLID STATE
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Passage|1 VideosSOME BASIC CONCEPTS OF CHEMISTRY
IIT-JEE PREVIOUS YEAR (CHEMISTRY)|Exercise Subjective Questions|2 Videos
Similar Questions
Explore conceptually related problems