A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLUTIONS
RESONANCE|Exercise Advabced Level Problems (PART-2)|35 VideosSOLUTIONS
RESONANCE|Exercise EXERCISE-3(PART-3)|30 VideosSOLUTION AND COLLIGATIVE PROPERTIES
RESONANCE|Exercise PHYSICAL CHEMITRY (SOLUTION & COLLIGATIVE PROPERTIES)|52 VideosSTEREOISOMERISM
RESONANCE|Exercise EXERCISE (PART III : PRACTICE TEST-2 (IIT-JEE (ADVANCED PATTERN))|22 Videos
Similar Questions
Explore conceptually related problems
RESONANCE-SOLUTIONS-Advabced Level Problems (PART-1)
- Elevation in b.p. of an aqueous urea solution is 0.52^(@), (K(b)=0.622...
Text Solution
|
- Insulin (C(2)H(10)O(5))(n) is dissolved in a suitable solvent and the ...
Text Solution
|
- What is the normal boiling point of the solution represented by the ph...
Text Solution
|
- Select correct statement ?
Text Solution
|
- Ratio at DeltaT(b)//K(b) of 6% AB(2) and 9%A(2)B(AB(2) and A(2)B both ...
Text Solution
|
- An aqueous solution of a solute AB has b.p. of 101.08^(@)C( AB is 100%...
Text Solution
|
- Density of 1M solution of a non-electrolyte C(6)H(12)O(6) is 1.18g//mL...
Text Solution
|
- Mole fraction of a non-electrolyte in aqueous solution is 0.07. If K(f...
Text Solution
|
- What is the normal freezing point of the solution represented by the p...
Text Solution
|
- Select correct staement :
Text Solution
|
- Some entropy change are represented infigure. Select correct entropy c...
Text Solution
|
- Total vapour pressure of mixture of 1 mol of volatile component A(P(A^...
Text Solution
|
- Water and chlorobenzene are immiscible liquids. Their mixture boils at...
Text Solution
|
- Relative decrease in vapour pressure of an aqueous NaCl is 0.167. Numb...
Text Solution
|
- Which statement comparing solutions with pure solvent is not correct
Text Solution
|
- A colligative property of a solution depends on the :
Text Solution
|
- Which has maximum freezing point?
Text Solution
|
- Van't Hoff factors of aqueous solutions of X,Y and Z are 2.8,1.8 and 3...
Text Solution
|
- Select correct statement :
Text Solution
|
- The vapour pressure of a pure liquid A is 40 mm Hg at 310 K. The vapou...
Text Solution
|
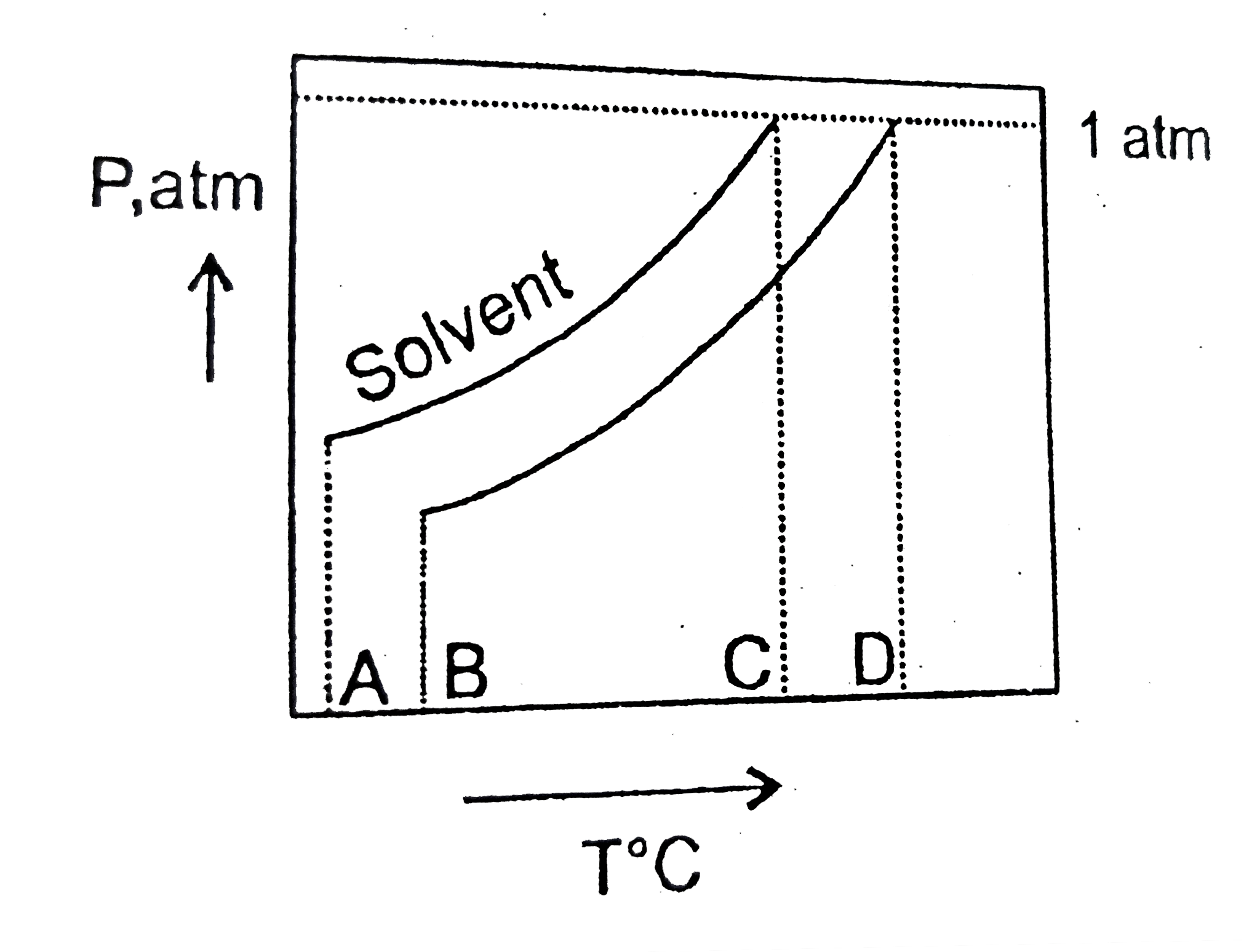
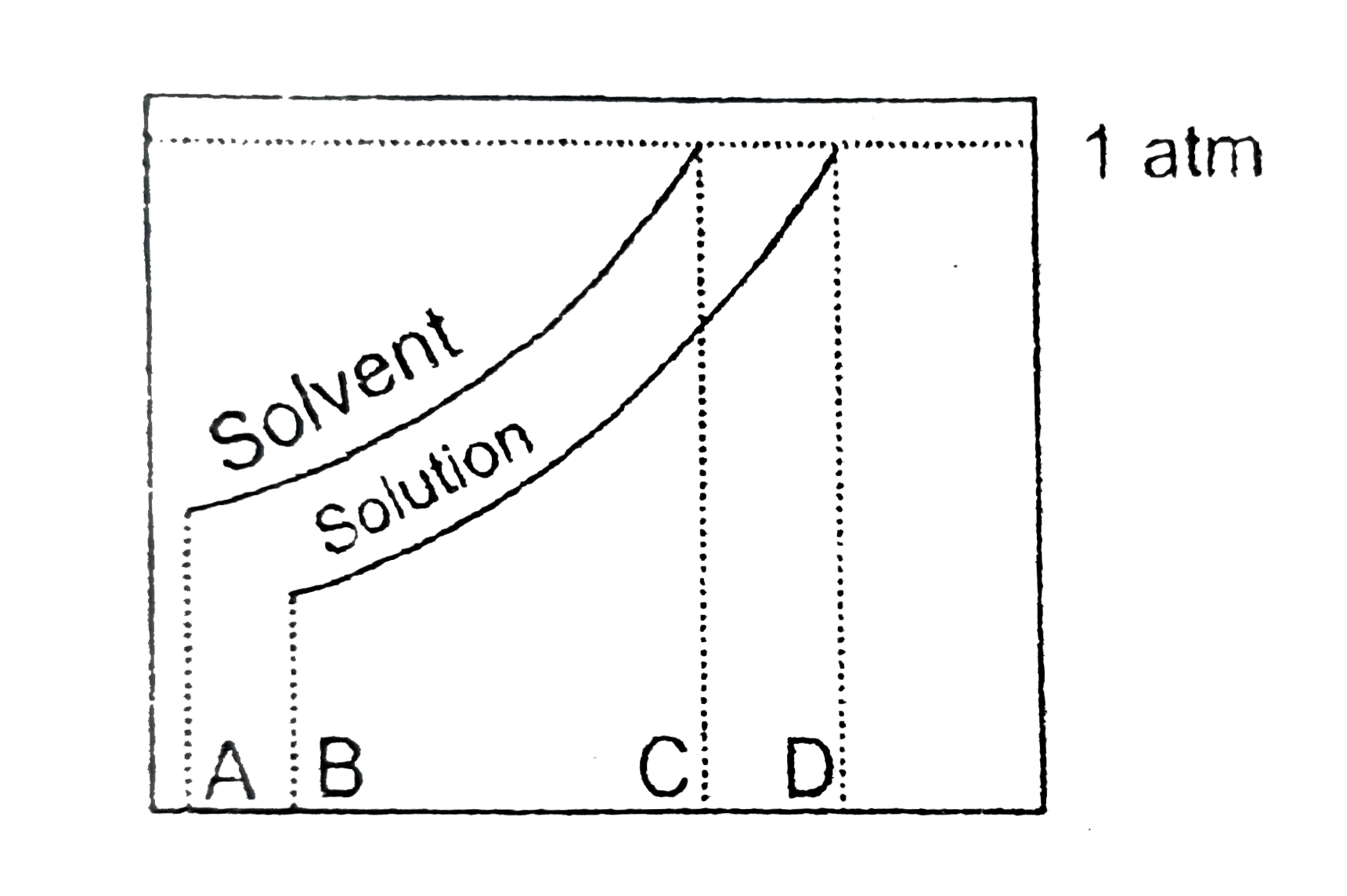 ltbegt Normal boiling point ot the solution is that temperature at which vapour pressure of solution equals to `1` atm
ltbegt Normal boiling point ot the solution is that temperature at which vapour pressure of solution equals to `1` atm