Let's consider a hypothetical planet `"pseudo Earth"` which is similar to our earth in several aspects. The similarities are
On pseudo earth:
(i) There are same number of elements as on our earth and they are known by the same name.
(ii) Pauli's exclusion principal, Hund's Rule and Aufbau principle are known to the people of pseudo earth in the same manner as we know on our earth.
(iii) They classify elements as representative, transition and inner- transition elements in the same manner as we classify on our earth.
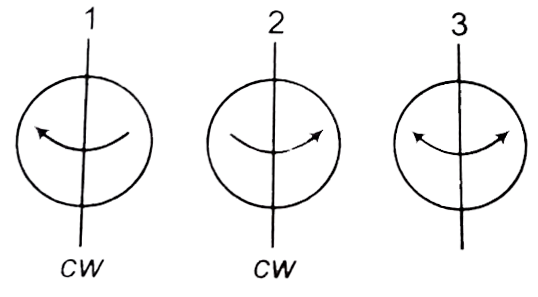
However, there is one basic difference in understanding the electrons, spin on these two earths. On our earth the electron can have only two spin directions. clock wise (1) and anti-clockwise (2), while on pseudo earth there is an additional possible value of spin quantum number called neutral spin (3) in which electron is believed to be fluctuating harmonically between clockwise and anti-clockwise directions, about its axis. Answer the following three question based on the above information.
The first noble gas on pseudo earth would be