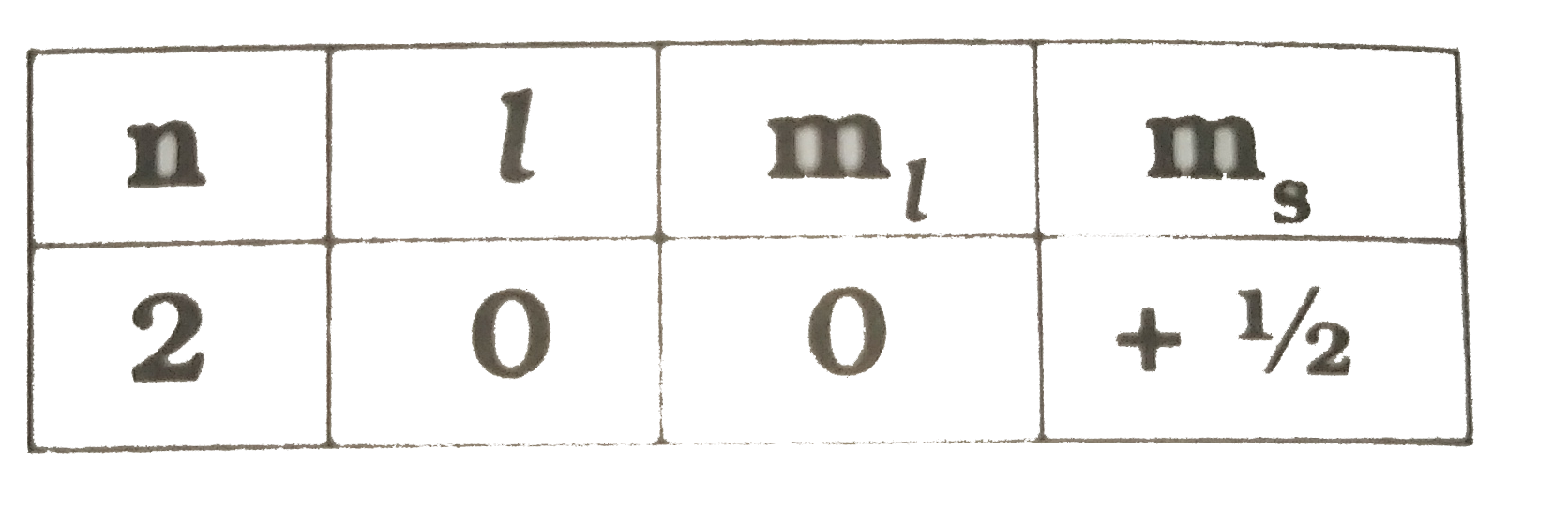Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise TEXTUAL LESSON PART ( INFORMATION SKILLS AND PROJECTS)|1 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise TEXTUAL LESSON PART (APPLICATION TO DAILY LIFE , CONCERN TO BIODIVERSITY)|1 VideosSTRUCTURE OF ATOM
VGS PUBLICATION-BRILLIANT|Exercise CREATIVE QUESTIONS FOR NEW MODEL EXAMINATION ( SECTION - IV 4 MARKS )|32 VideosREFLECTION AND REFRACTION
VGS PUBLICATION-BRILLIANT|Exercise EXERCISE|24 VideosSUMMATIVE ASSESSMENT
VGS PUBLICATION-BRILLIANT|Exercise SUMMATIVE ASSESSMENT|28 Videos
Similar Questions
Explore conceptually related problems