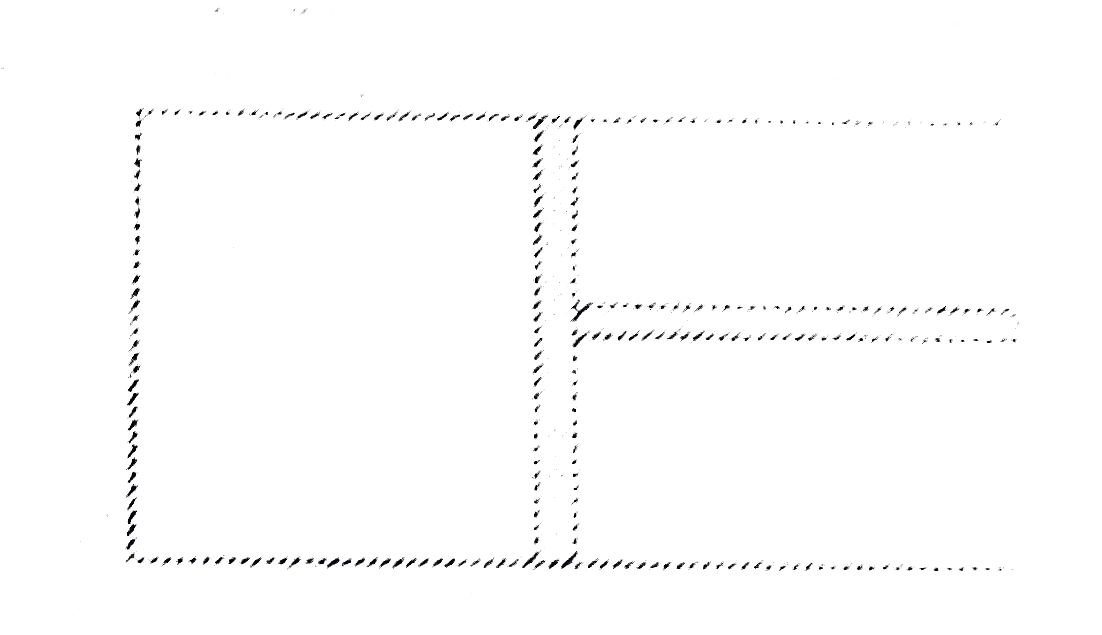
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
CENGAGE PHYSICS|Exercise 11|3 VideosTHERMODYNAMICS
CENGAGE PHYSICS|Exercise 12|3 VideosTHERMODYNAMICS
CENGAGE PHYSICS|Exercise 9|3 VideosSUPERPOSITION AND STANDING WAVES
CENGAGE PHYSICS|Exercise Comprehension Type|5 VideosTRANSMISSION OF HEAT
CENGAGE PHYSICS|Exercise Single correct|9 Videos
Similar Questions
Explore conceptually related problems