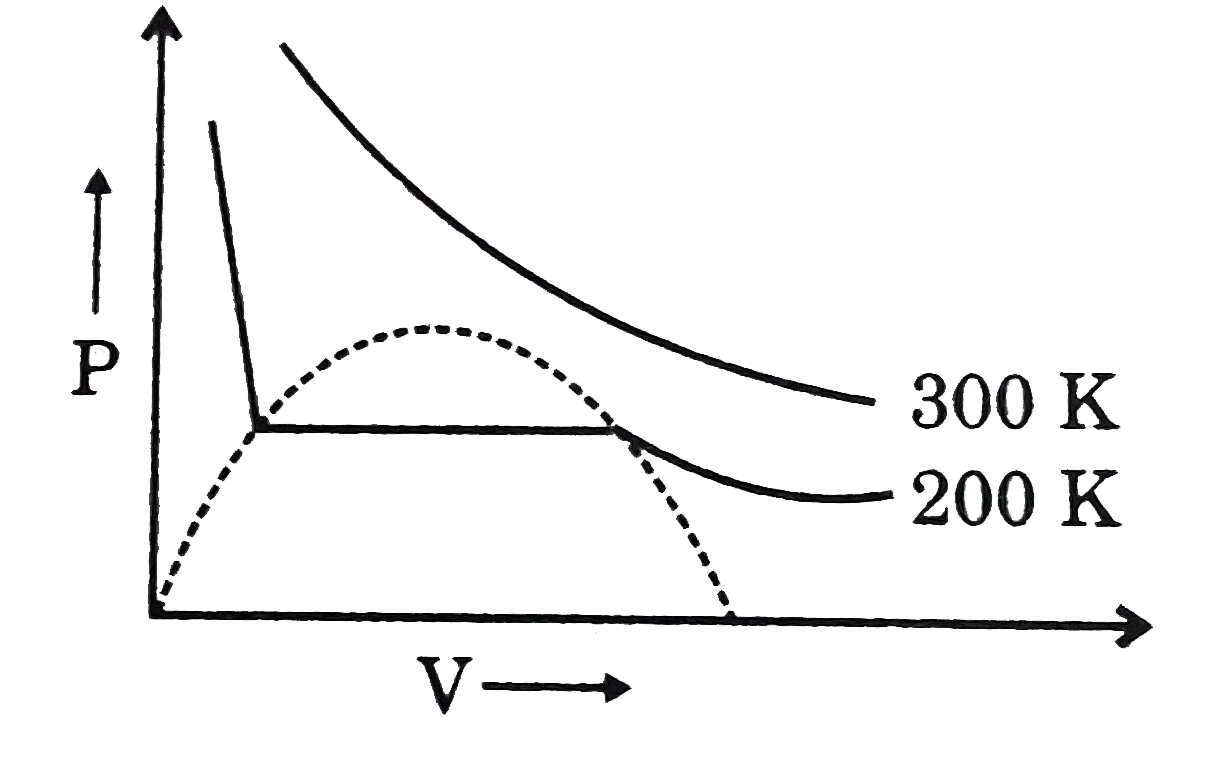
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Gaseous State|39 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise C. Atomic Sturcture|1 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Thermodynamics|45 VideosF-BLOCK ELEMENTS
GRB PUBLICATION|Exercise Subjective type|7 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 Videos
Similar Questions
Explore conceptually related problems