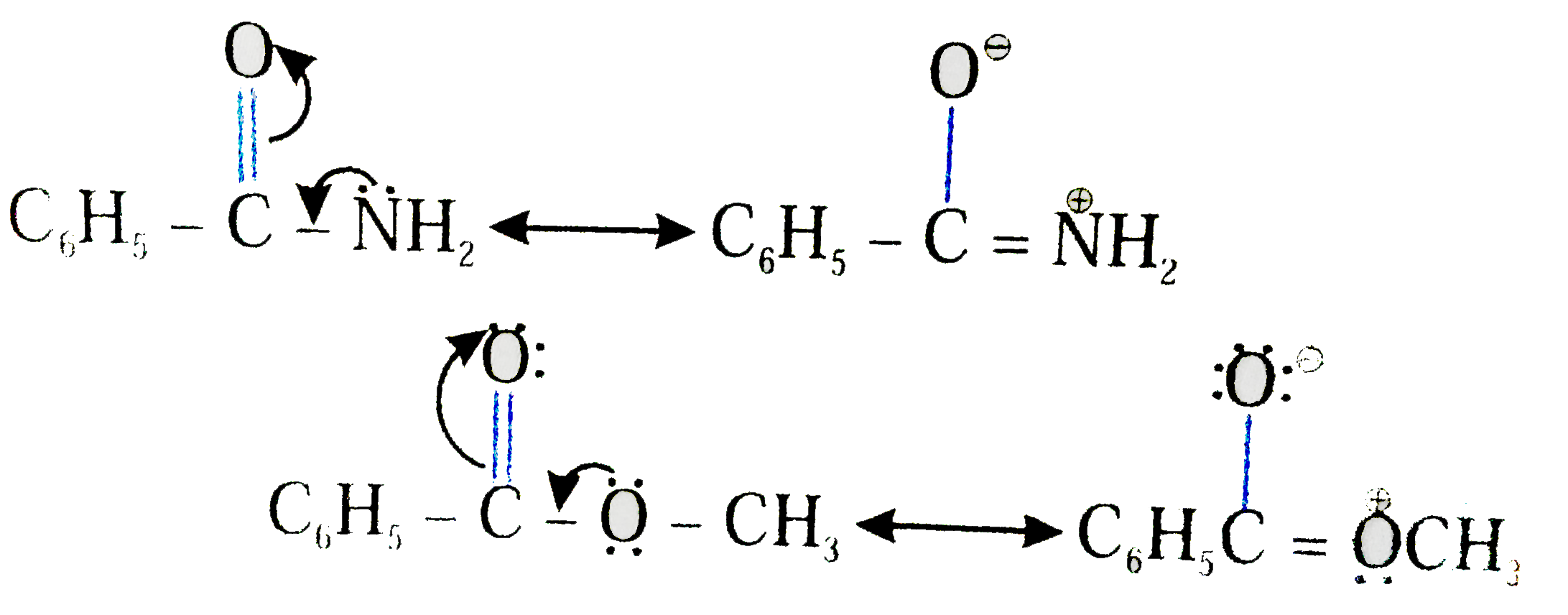Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NARAYNA-CARBOXYLIC ACID-All Questions
- The end product of the following reaction
Text Solution
|
- What is the most likely product form of the following reaction ? CH(...
Text Solution
|
- Explain why benzamide is less readily hydrolysed than methyl Benzoate.
Text Solution
|
- Starting with parabenzoquinone how will you obtain 1,2,-4,-triacetoxy ...
Text Solution
|
- Compared to alcohol explain why phenols cannot be converted into easte...
Text Solution
|
- Write the mechanism for the following reaction
Text Solution
|
- How will you prepare hippuricated from Benzoylchloride ?
Text Solution
|
- The acid present in red ants is
Text Solution
|
- beta-chlorobutyric acid is name in IUPAC
Text Solution
|
- The general formula of C(n)H(2n)O(2) could be for open chain
Text Solution
|
- CH(3)Cl overset(KCN) A A overset(H(2)O^(+)) to B(final product) In t...
Text Solution
|
- CH(3)OH overset((i) X)underset((ii) Rh, Delta)toCH(3)CO OH. In this re...
Text Solution
|
- In vinegar the concentration of acetic acid is nearly
Text Solution
|
- Which of the following has highest boiling point ?
Text Solution
|
- The products formed when PCl(5) reacts with acetic acid are
Text Solution
|
- Which of the following reduces carboxylic acid directly to primary alc...
Text Solution
|
- Number of hydrogen bonds present between two acetic acid molecules whe...
Text Solution
|
- Which is false about acetic acid
Text Solution
|
- In the following reaction, X and Y are respectively. CH(3)CO OH+NH(3...
Text Solution
|
- Assertion (A) : CH(3)CN on hydrolysis gives Acetic Acid Reason (R) :...
Text Solution
|