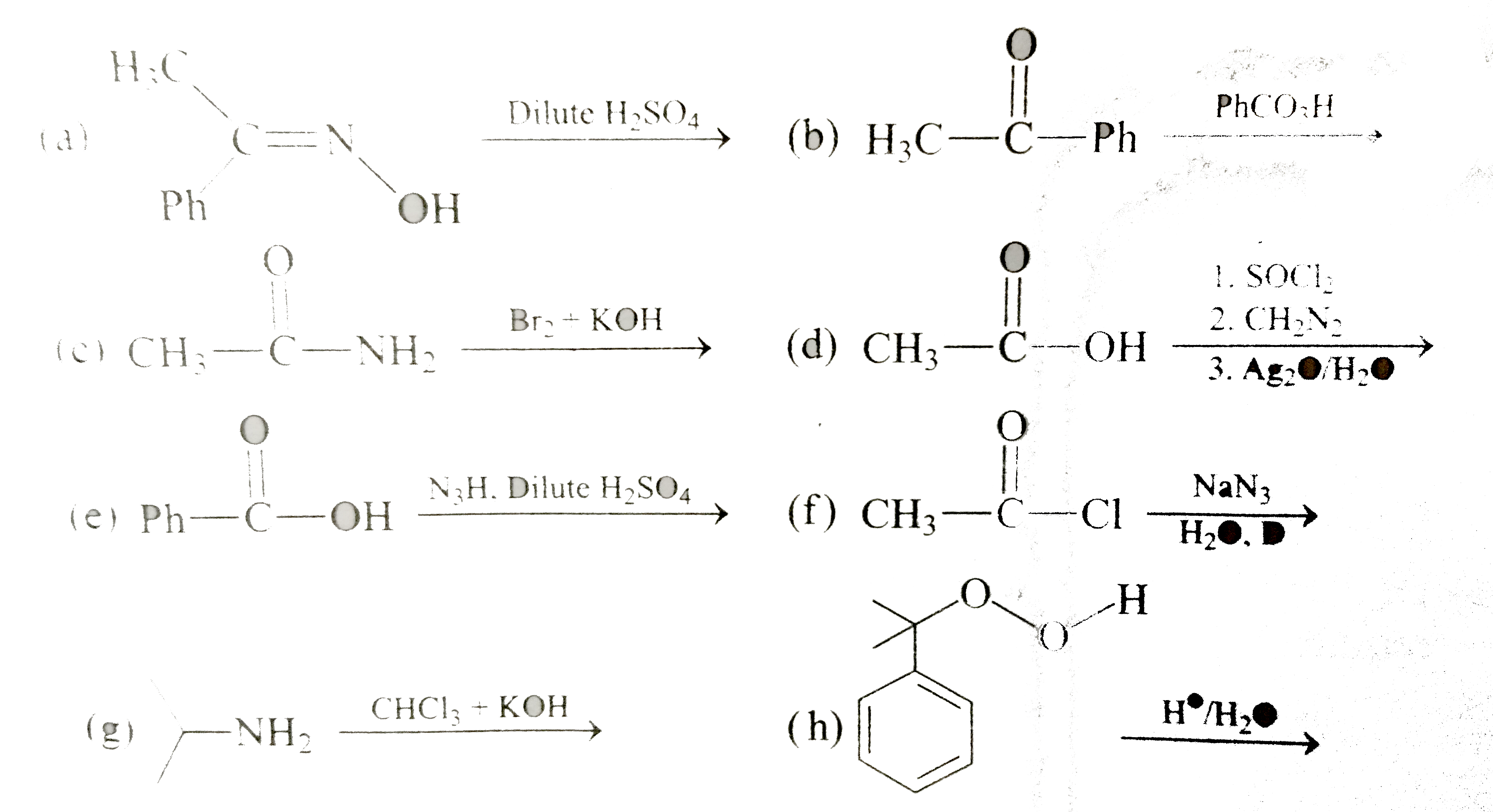
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AMINES-Integer Answer Type Problems
- Find out number of reaction which involve electron deficient nitrogen ...
Text Solution
|
- Examine the structal formules of following compounds and identify how ...
Text Solution
|
- Of the following amines how many can give carbyl amine reaction?
Text Solution
|
- Of the following reactions, how many reaction, are used for the prepa...
Text Solution
|
- Of the following amines how many can be separately by Hofmann's mustar...
Text Solution
|