A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
NARAYNA-PERIODIC TABLE -All Questions
- The periodicity is related to the electronic configuration. That is, a...
Text Solution
|
- The periodicity is related to the electronic configuration. That is, a...
Text Solution
|
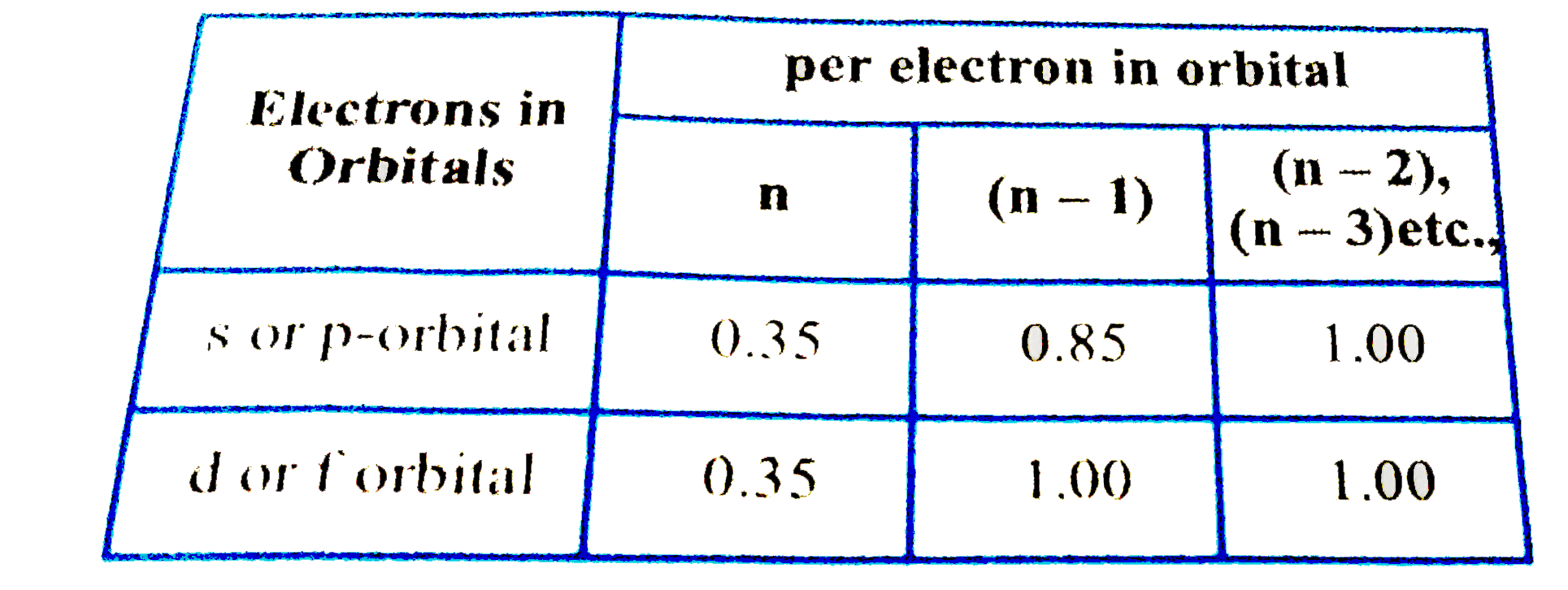
- According to I.C slater effective nuclear charge, Z^(**), due to scree...
Text Solution
|
- According to I.C slater effective nuclear charge, Z^(**), due to scree...
Text Solution
|
- According to I.C slater effective nuclear charge, Z^(**), due to scree...
Text Solution
|
- The number of elements among the followingb which have lower electro n...
Text Solution
|
- The first four successive ionization energies for an element are 6 , 1...
Text Solution
|
- The number of species among the following , having insert gas configu...
Text Solution
|
- The number of elements among the following atomic numbers that are p b...
Text Solution
|
- Among the following oxides, how many of them are amphoteric oxides? ...
Text Solution
|
- The no. of elements which are more electropositive than 'Fe' in the f...
Text Solution
|
- The no. of paramagnetic species in the following . Na^(+),Cl^(-), Sn^(...
Text Solution
|
- 1.0 g of Mg atom ("atomic mass" = 24.0 "amu") in the vapour phase abso...
Text Solution
|
- The ionisation energy of Li is 500 KJ/mole. The amount of energy requi...
Text Solution
|
- The first IP lithium is 5.41eV and electron gain enthalpy of Cl is -3....
Text Solution
|
- How many of the following statements are correct according to Mendelee...
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|
- Match the following columns
Text Solution
|