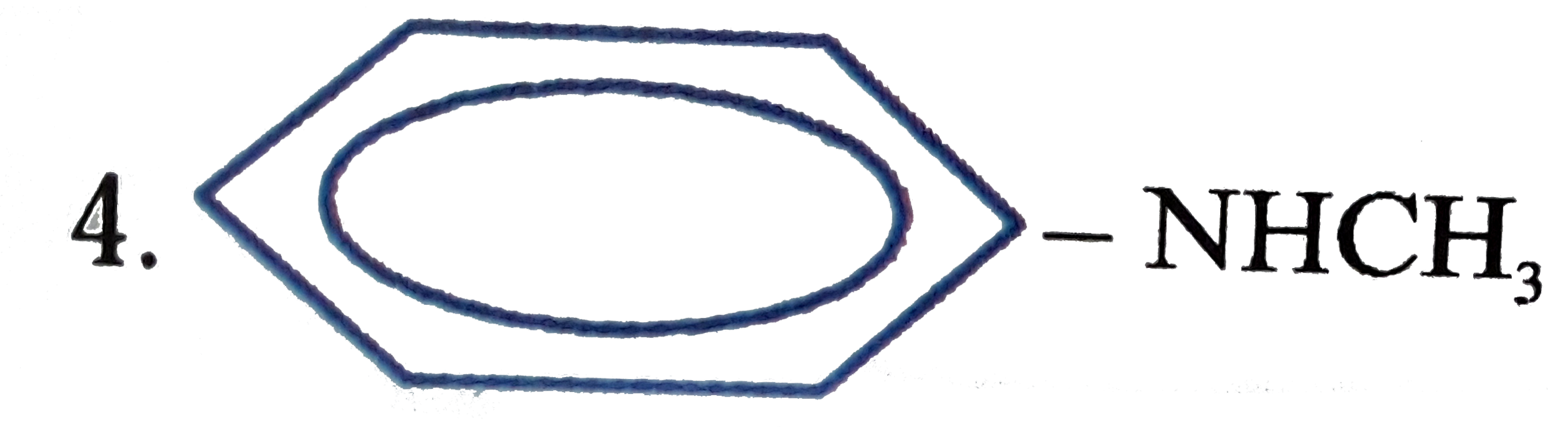
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level -IV|30 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Level -I (H.w)|19 VideosAMINES & DIAZO COMPOUNDS
NARAYNA|Exercise Previous year|9 VideosALDEHYDES, KETONES & CARBOXYLIC ACIDS
NARAYNA|Exercise CHECK YOUR GRASP|6 VideosBIOMOLECULES
NARAYNA|Exercise EXERCISE-4 (NCERT EXAMPLAR PROBLEMS)|12 Videos
Similar Questions
Explore conceptually related problems
NARAYNA-AMINES & DIAZO COMPOUNDS-Level -III
- Maximum pKb value of
Text Solution
|
- Which of the following orders is true regarding the basic nature of NH...
Text Solution
|
- Hoffmann degradation of m-bromobenzamide gives
Text Solution
|
- An aromatic amine (X) was treated with alcoholic potash and another co...
Text Solution
|
- Which of the following would not react with benzene sulphonyl chloride...
Text Solution
|
- Fluorobenzene (C(6)H(5)F) can be synthesized in the laboratory .
Text Solution
|
- CH3 CH2 Cl overset ("NaOH")to X overset ("Ni//H2") to Y overset("Aceti...
Text Solution
|
- C6 H6 overset("Conc." HNO3) underset("Conc." H2 SO4 . 363K) to X overs...
Text Solution
|
- In the given reaction CH(3)-CH(2)-overset(CH(3))overset(|)underset(C...
Text Solution
|
- Match the following columns
Text Solution
|
- In the diazotisation of anline with sodium nitrite and hydrochloride a...
Text Solution
|
- The compound which on rection with aqueous nirous acid at low temperat...
Text Solution
|
- Which of the following orders is correct regarding basicity of indicat...
Text Solution
|
- Towards electrophilic substitution, the most reactive is
Text Solution
|
- Z' in the following sequence of reaction is C6 H6 overset(HNO3 // H2 S...
Text Solution
|
- The reaction of chloroform with alcoholic KOH and p- toluidine forms
Text Solution
|
- In the Hofmann's method for separation of 1^@, 2^@ and 3^@ amines, the...
Text Solution
|
- Libermann's nitroso reaction is used for testing
Text Solution
|
- Amine which will not respond to Benzoylation reaction is
Text Solution
|