A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 18-PHYSICS
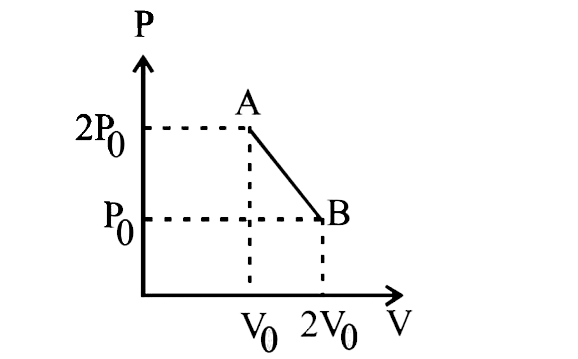
- n moles of an ideal gas undergoes a process AtoB as shown in the figur...
Text Solution
|
- A door 1.6 m wide requires a force of 1 N to be applied at the free an...
Text Solution
|
- A second pendulum is moved to moon where acceleration due to gravity i...
Text Solution
|
- For a transistor the current amplification factor is 0.8 The transisto...
Text Solution
|
- A magnetic dipole is acted upon by two magnetic fields with inclined t...
Text Solution
|
- An ideal gas is expanding such that PT^(2)= constant. The coefficient ...
Text Solution
|
- A student is performing the experiment of resonance column. The diamet...
Text Solution
|
- In a Young's double slit experiment, 12 fringes are observed to be for...
Text Solution
|
- A wire of length l has a resistance R. If half of the length is stretc...
Text Solution
|
- Find the Q value of the reaction P + .^(7) Li rarr .^(4) He +.^(4) He....
Text Solution
|
- A wheel having mass m has charges +q and -q on diametrically opposite ...
Text Solution
|
- If the following atoms and molecules for the transition from n = 2 to ...
Text Solution
|
- The beta- activity of a sample of CO(2) prepared form a contemporary ...
Text Solution
|
- The gravitational field in a region is given by vecg=(5hati+12hatj)"N ...
Text Solution
|
- Time taken by the particle to reach from A to B is t. Then the distanc...
Text Solution
|
- Two particles of charges +Q and -Q are projected from the same point w...
Text Solution
|
- In the adjacent circuit, the instantaneous current equation is
Text Solution
|
- Oxygen gas is made to undergo a process in which its molar heat capaci...
Text Solution
|
- A glass capillary tube is of the shape of a truncated cone with an ape...
Text Solution
|
- Two electric lamps A and B radiate the same power. Their filaments hav...
Text Solution
|