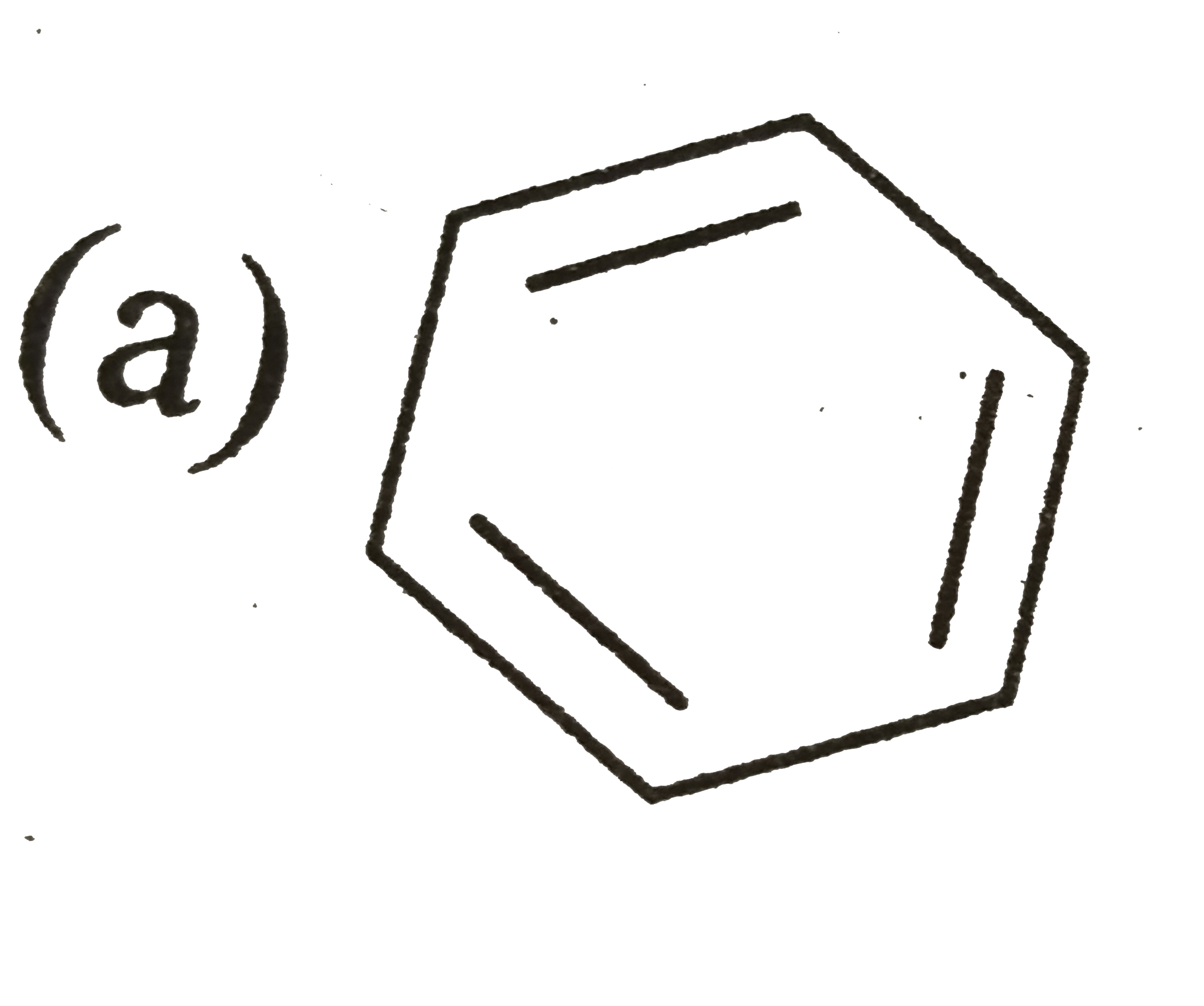
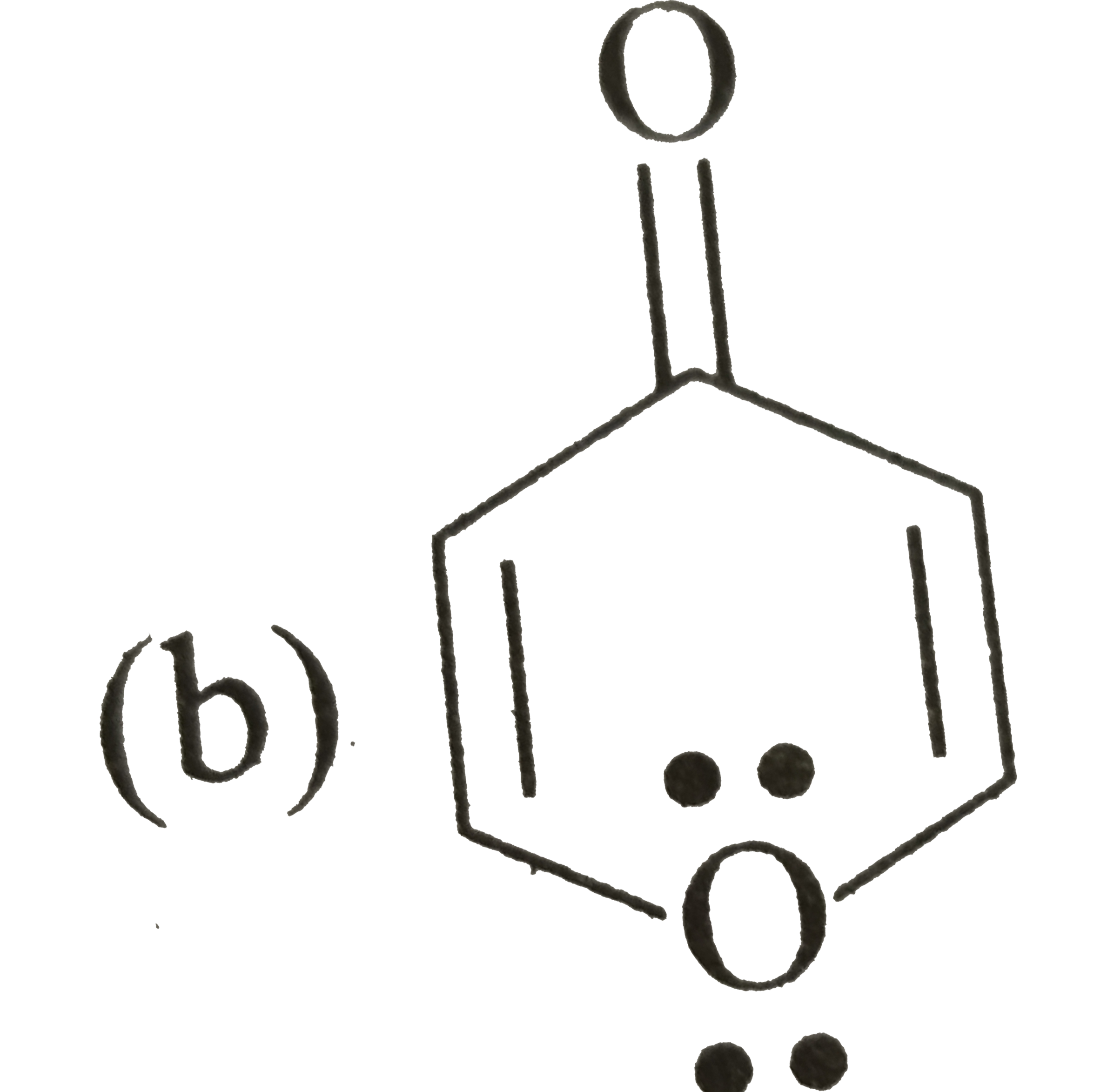
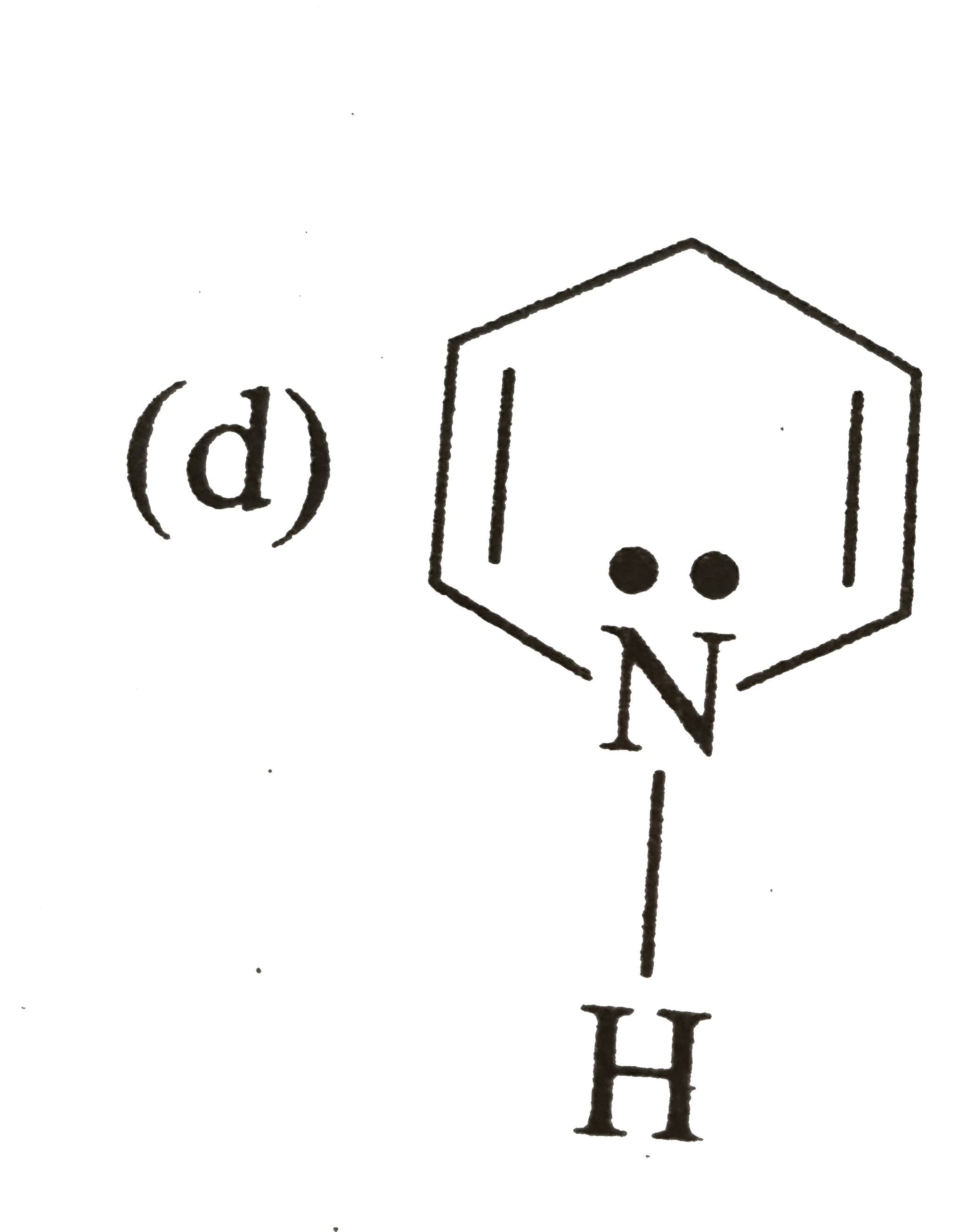
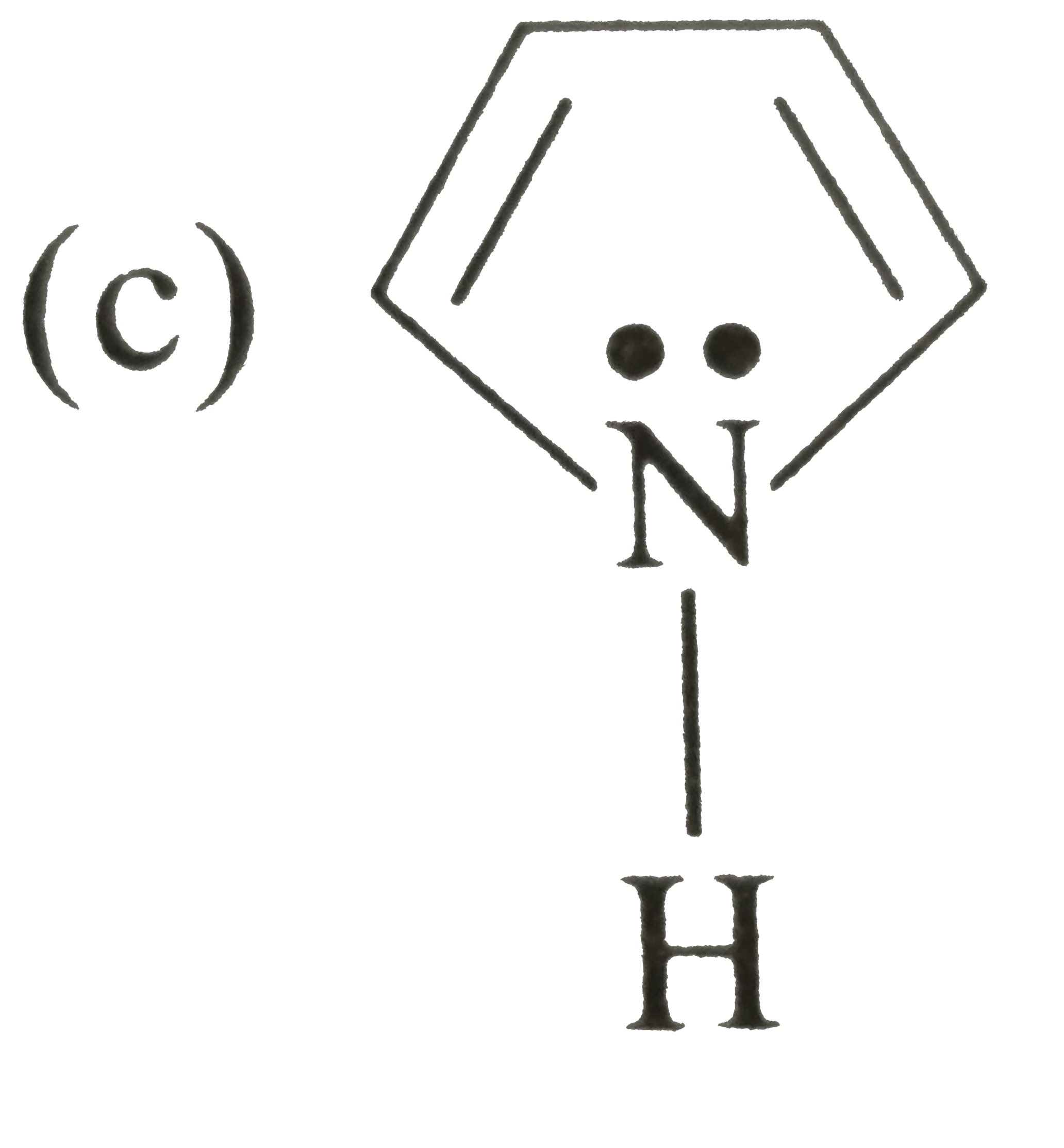A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
AROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Match The Column|5 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|10 VideosAROMATIC HYDROCARBONS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.35)|10 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 VideosBIOMOLECULES
HIMANSHU PANDEY|Exercise Match The Column|6 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-AROMATIC HYDROCARBONS-Linked Comprehension Type
- For any compound to be aromatic, compound should follow a given set...
Text Solution
|
- For any compound to be aromatic, compound should follow a given set...
Text Solution
|
- For any compound to be aromatic, compound should follow a given set...
Text Solution
|
- For any compound to be aromatic, compound should follow a given set...
Text Solution
|
- Directing nature of substuted aromatic compound is decided by stabil...
Text Solution
|
- Directing nature of substuted aromatic compound is decided by stabil...
Text Solution
|
- Directing nature of substuted aromatic compound is decided by stabil...
Text Solution
|
- If aromatic ring is substitued by more than groups then electrophilic...
Text Solution
|
- If aromatic ring is substitued by more than groups then electrophilic...
Text Solution
|
- If aromatic ring is substitued by more than groups then electrophilic...
Text Solution
|
- A benzene ring deactived by strong and moderate electrons withdrawi...
Text Solution
|
- A benzene ring deactived by strong and moderate electrons withdrawi...
Text Solution
|
- A benzene ring deactived by strong and moderate electrons withdrawi...
Text Solution
|
- For a typical nucleophilic aromatic subsitution reaction to take pl...
Text Solution
|
- For a typical nucleophilic aromatic subsitution reaction to take pl...
Text Solution
|
- For a typical nucleophilic aromatic subsitution reaction to take pl...
Text Solution
|
- Examine given sequence of reaction carefully : overset(Sn+HCl)to A...
Text Solution
|
- Examine given sequence of reaction carefully : overset(Sn+HCl)to A...
Text Solution
|
- Examine given sequence of reaction carefully : overset(Sn+HCl)to A...
Text Solution
|
- There is a way to reduce benzene derivatives to the corresponding 4...
Text Solution
|