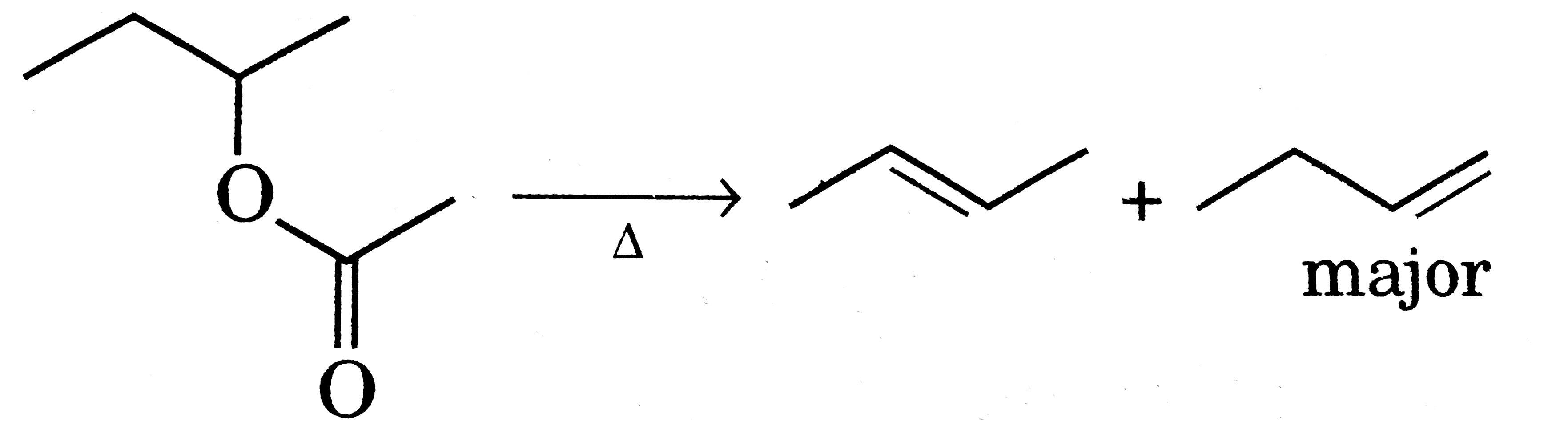
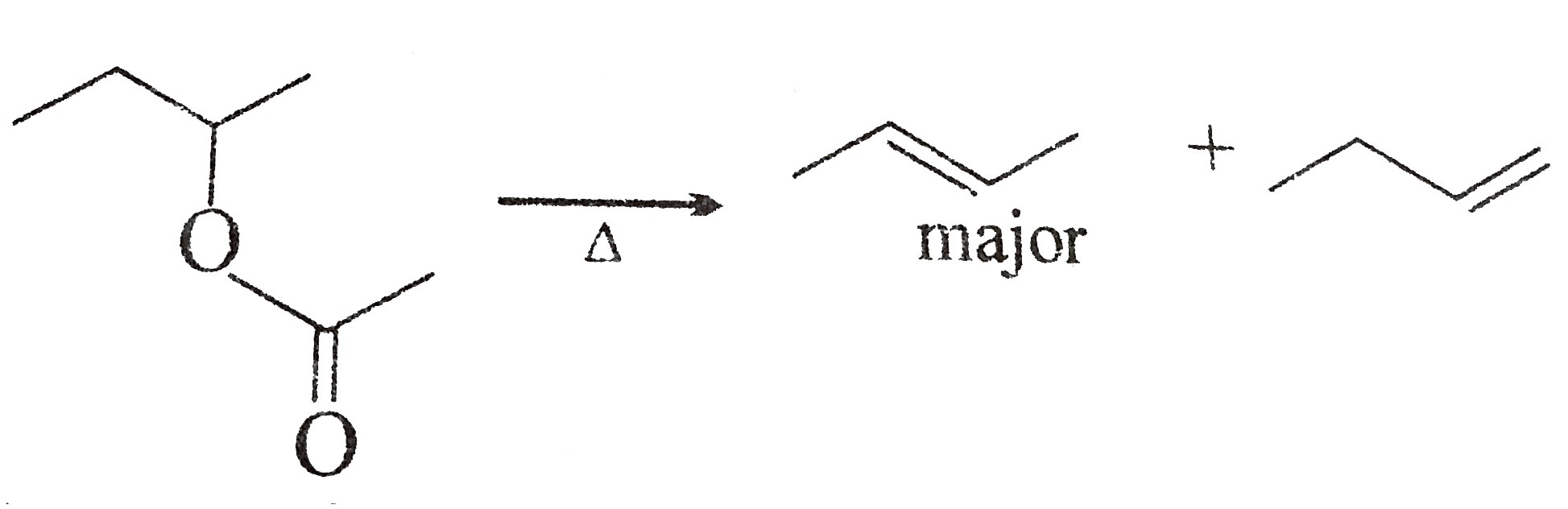A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Multiple Objective Type|56 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Comprehension Type-1|3 VideosHYDROCARBON (ALIPHATIC)
GRB PUBLICATION|Exercise Previous years jee questions|28 VideosGRAPHICAL INTERPRETATION
GRB PUBLICATION|Exercise Subjective Type|23 VideosHYDROGEN AND ITS COMPOUNDS
GRB PUBLICATION|Exercise Subjective type|8 Videos
Similar Questions
Explore conceptually related problems
GRB PUBLICATION-HYDROCARBON (ALIPHATIC)-Reasoning Type
- Statement-I: Statement-II: 2-butene is more stable than -2butene as ...
Text Solution
|
- Statement-1 : Statement-2 : In reactions involving epoxidation f...
Text Solution
|
- Statement-I: Statement-II:Birch reduction is anti addition.
Text Solution
|
- Statement-1 : Statement - 2 : P(1) is having 6 alpha-H while P(2)...
Text Solution
|
- Statement-I, underset("Major product")(Me-overset(OH)overset(|)(C)H...
Text Solution
|
- Statement-1 : Rate of electrophilic addition on alkene Higt HBr gt HCI...
Text Solution
|
- Statement-1 : Propane on photochlorination gives 2-chlro propane as ma...
Text Solution
|
- Statement-1 : The major product formed by the electrolysis of aqueous ...
Text Solution
|
- Statement-1 : When 1 mol of Ph(3) overset(.)C are generated together ...
Text Solution
|
- Statement-1 : Ether solvent used in Wurtz reaction must be absolutely ...
Text Solution
|
- Statement-1 : Cl(2) reacts with islobutane in hv to produce 3^(@) buty...
Text Solution
|
- Assertion: Addition of Br(2) to 1-butane gives two optical isomers. ...
Text Solution
|
- Statement I: 1-Butene on reaction with HBr in the presence of a peroxi...
Text Solution
|
- Addition of bromine to trans-2-butene yields meso-2,3-dibromobutane. ...
Text Solution
|
- Assertion : Dimethyl sulphide is commonly used for the reduction of a...
Text Solution
|