Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
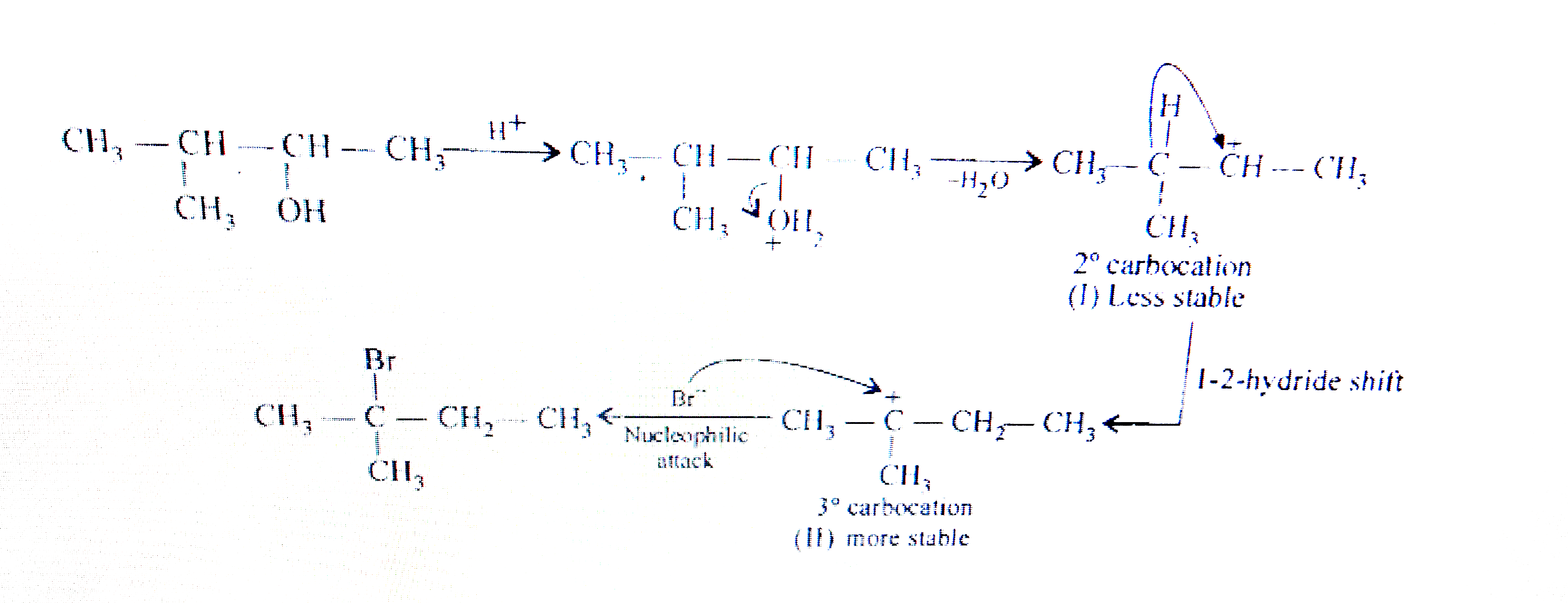
- When 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- CH(3)-underset(CH(3)) underset(|) (C)= CH(2) underset("Peroxide") over...
Text Solution
|
- When 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- जब 3-मेथिल ब्यूटेन-2-ऑल की क्रिया HBr से करायी जाती है, तो निम्न क्रिय...
Text Solution
|
- 3-मेथिल ब्यूटेन-2-ऑल को HBr से अभिकृत कराने पर निम्नलिखित अभिक्रिया हो...
Text Solution
|
- The major product formed in the reaction is: CH(3)-underset(H)unders...
Text Solution
|
- When 3-methylbutan-2-ol is treated with HBr, the following reaction ta...
Text Solution
|
- The IUPAC name of CH(3)-underset(OH)underset(|)(CH)-CH(2)-CH(2)-CH(2...
Text Solution
|
- (a) निम्न यौगिको के IUPAC नाम दीजिए। (i) overset(4)(CH(3)) – underse...
Text Solution
|