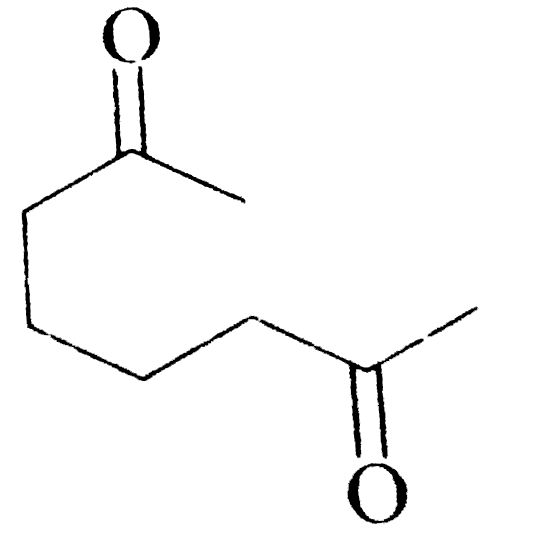
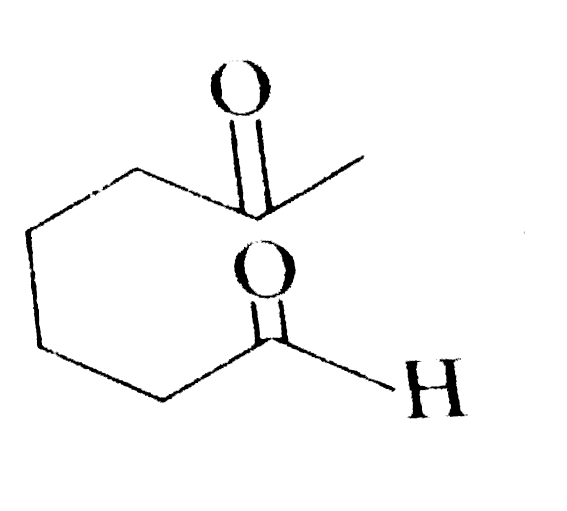
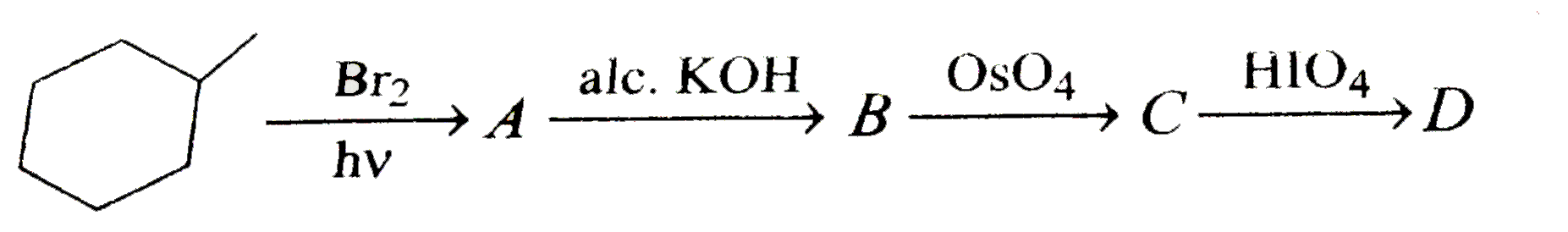A
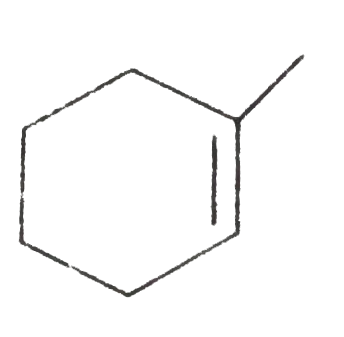
B
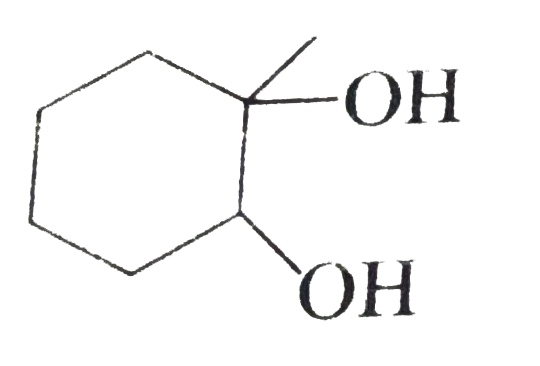
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise Match The Column|8 VideosALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise Integer Answer Type Problems|10 VideosALCOHOLS AND ETHERS
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.50)|25 VideosAMINES
HIMANSHU PANDEY|Exercise Subjective Type Problems|5 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-ALCOHOLS AND ETHERS-Linked Comprehension Type
- Althought epoxides do not contain a good leaving group, they contains ...
Text Solution
|
- Althought epoxides do not contain a good leaving group, they contains ...
Text Solution
|
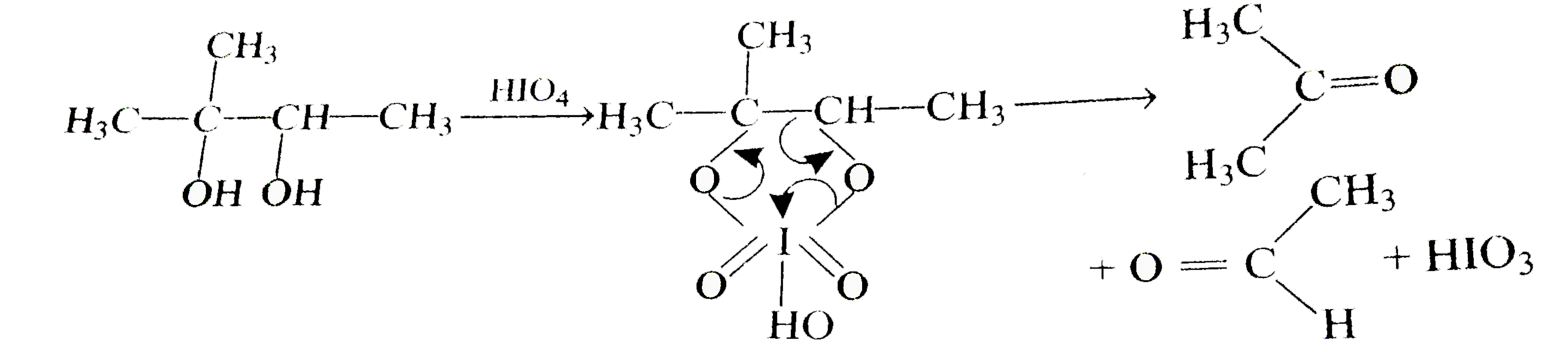
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- 1,2-diols are oxidised to ketones or aldehydes by periodic acid HIO(4)...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- Carbon oxygen double bond are easily reduced by NaBH(4) or LiAlH(4). T...
Text Solution
|
- An organic compound (A) on treatment with CHCl(3) and KOH gives (Y) an...
Text Solution
|
- An organic compound (A) on treatment with CHCl(3) and KOH gives (Y) an...
Text Solution
|
- An organic compound (A) on treatment with CHCl(3) and KOH gives (Y) an...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- A tertiary alcohol (H) upon acid-catalysed dehydration gives a product...
Text Solution
|
- Alcohols are converted to tosylates by treatment with p-toluence sulfo...
Text Solution
|
- Alcohols are converted to tosylates by treatment with p-toluence sulfo...
Text Solution
|
- Alcohols are converted to tosylates by treatment with p-toluence sulfo...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|
- Acid catalysed conversation of 1,2-diol or vicinal, into carbonyl comp...
Text Solution
|