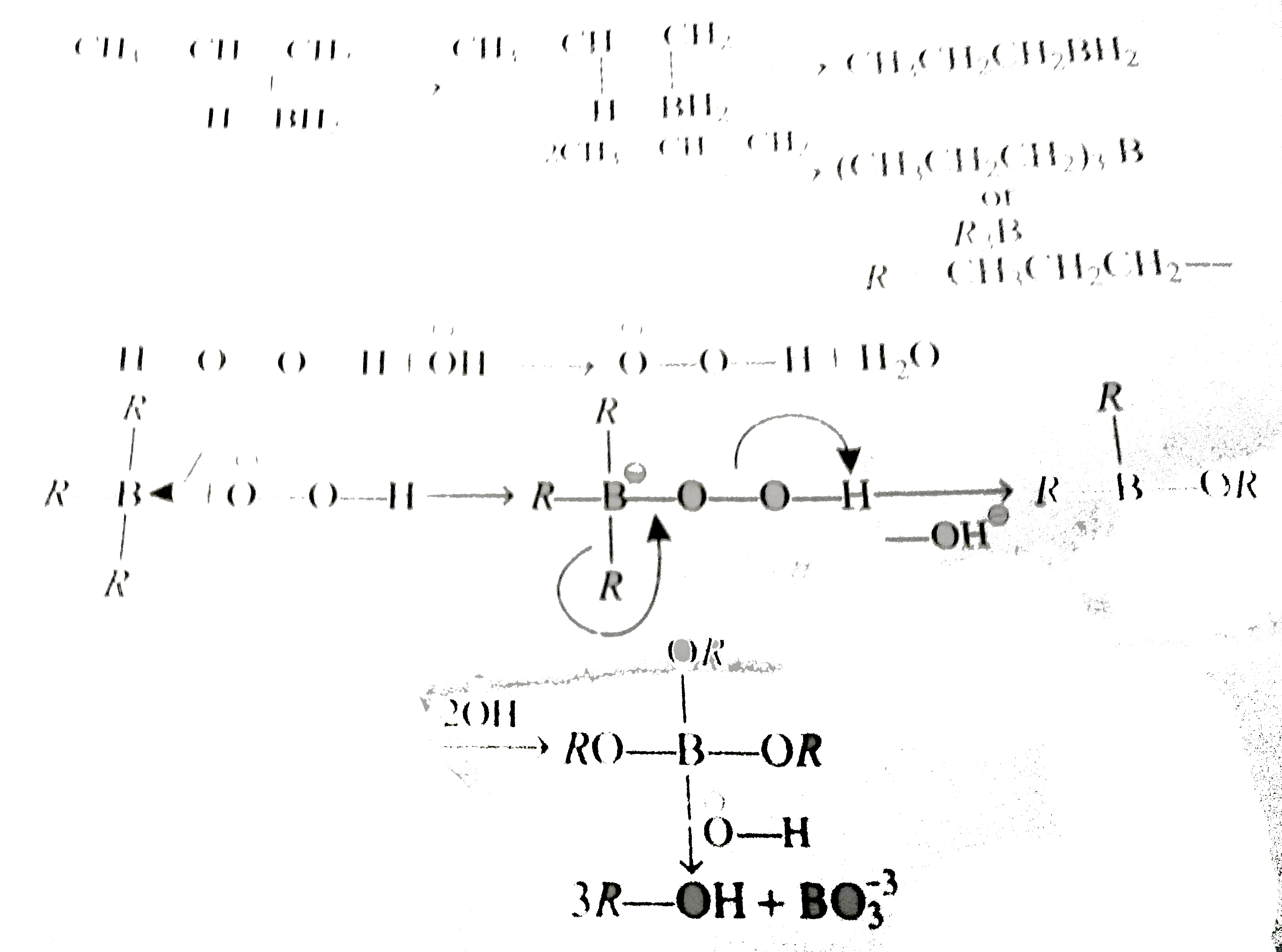
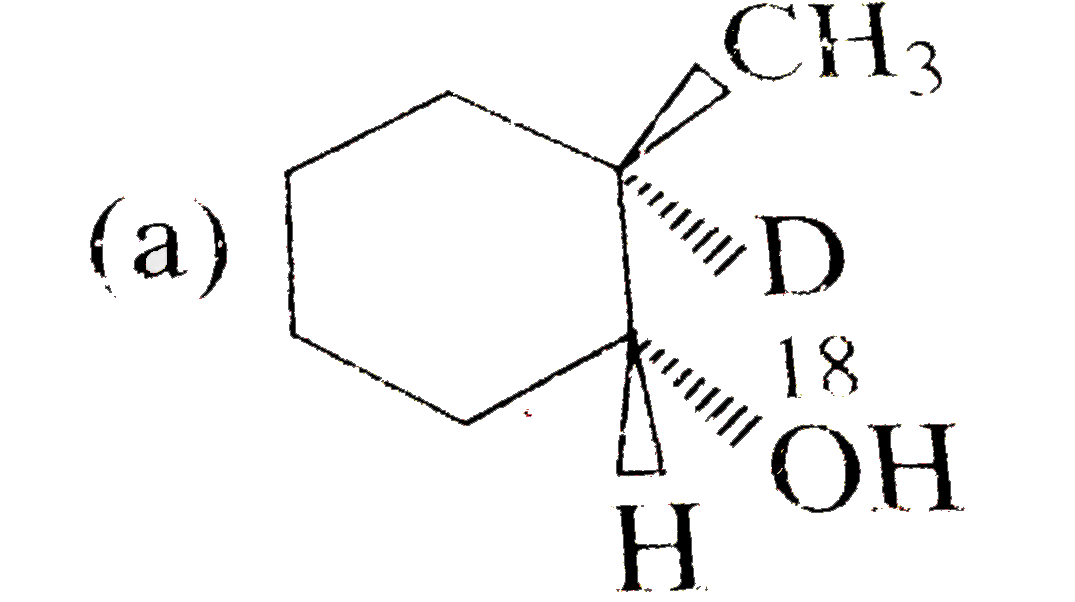
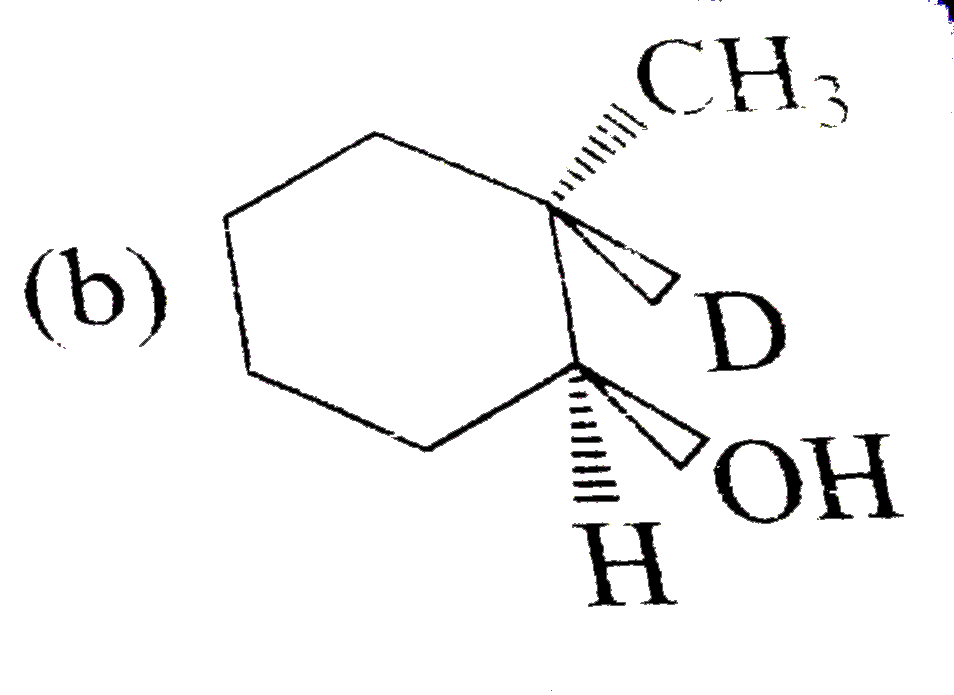
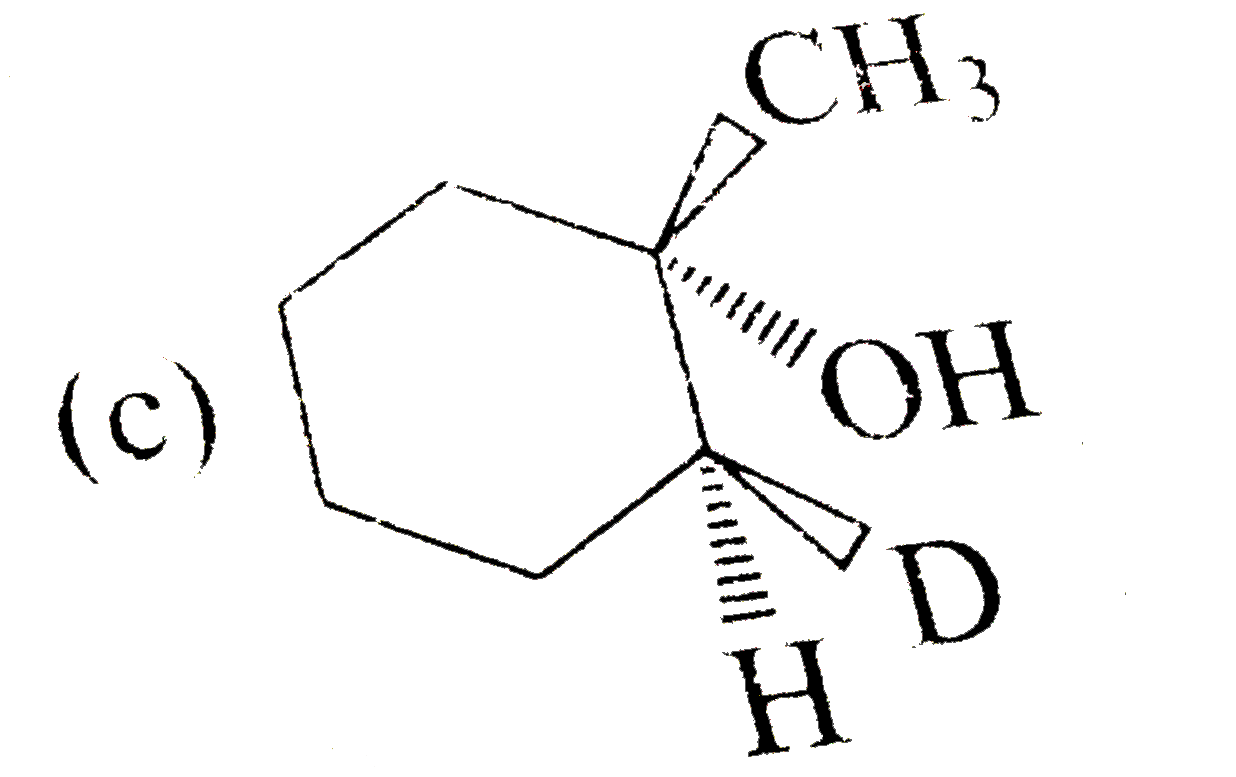
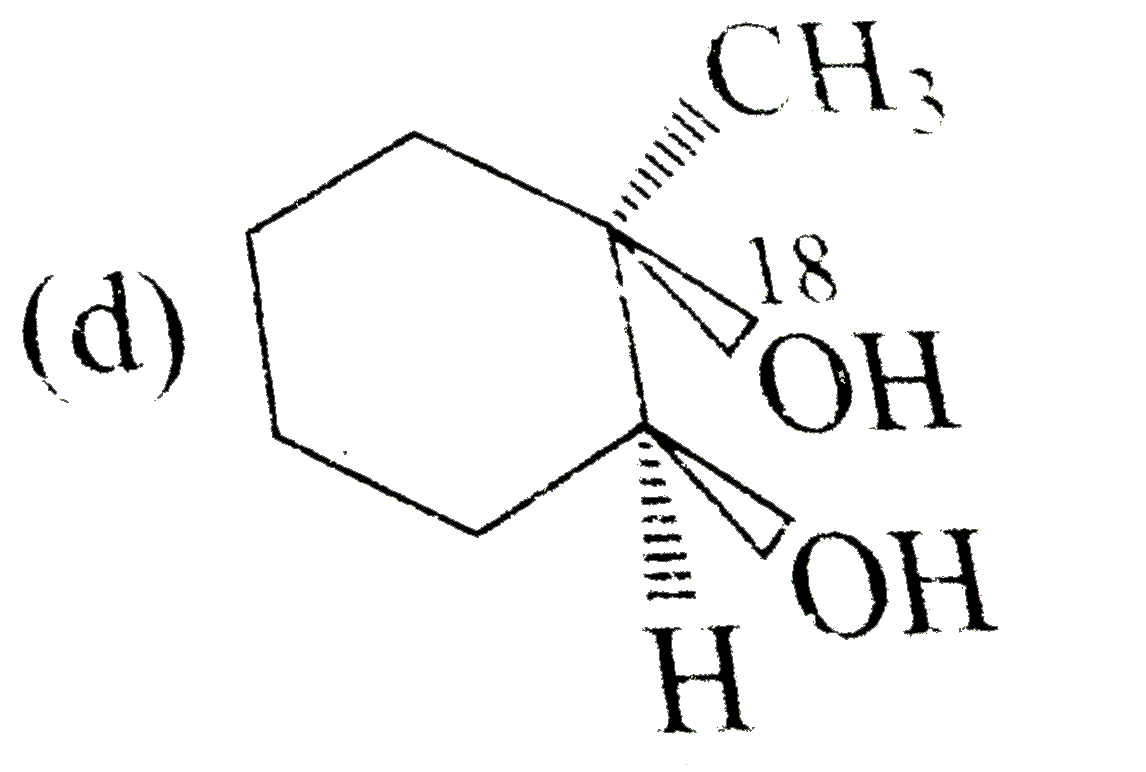
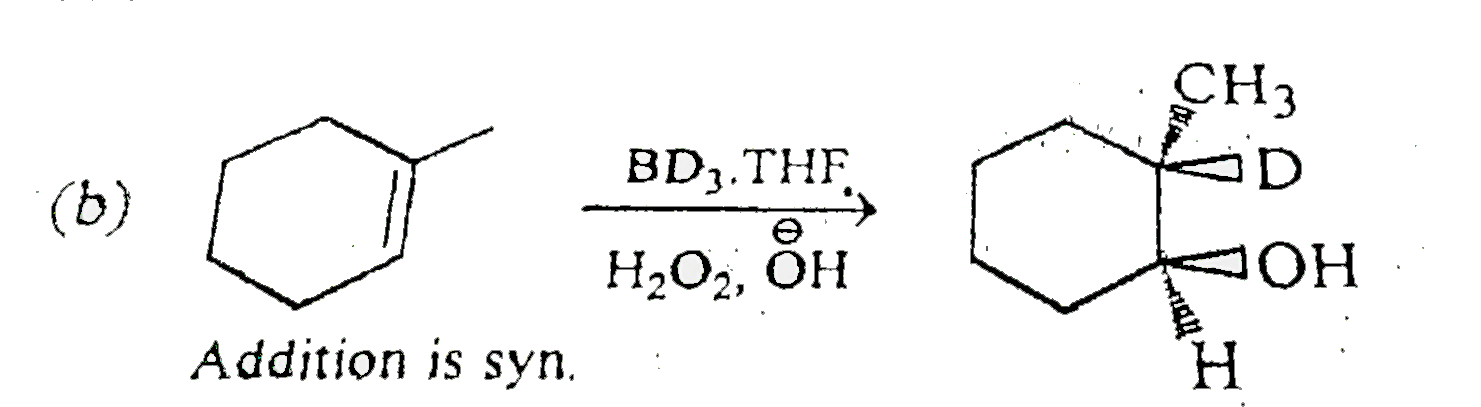
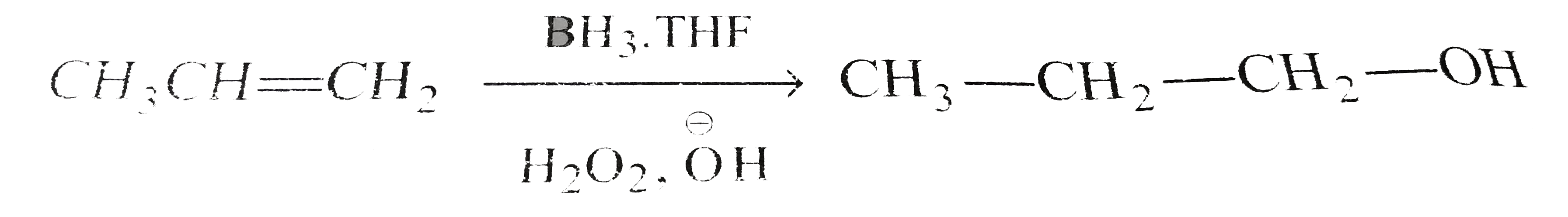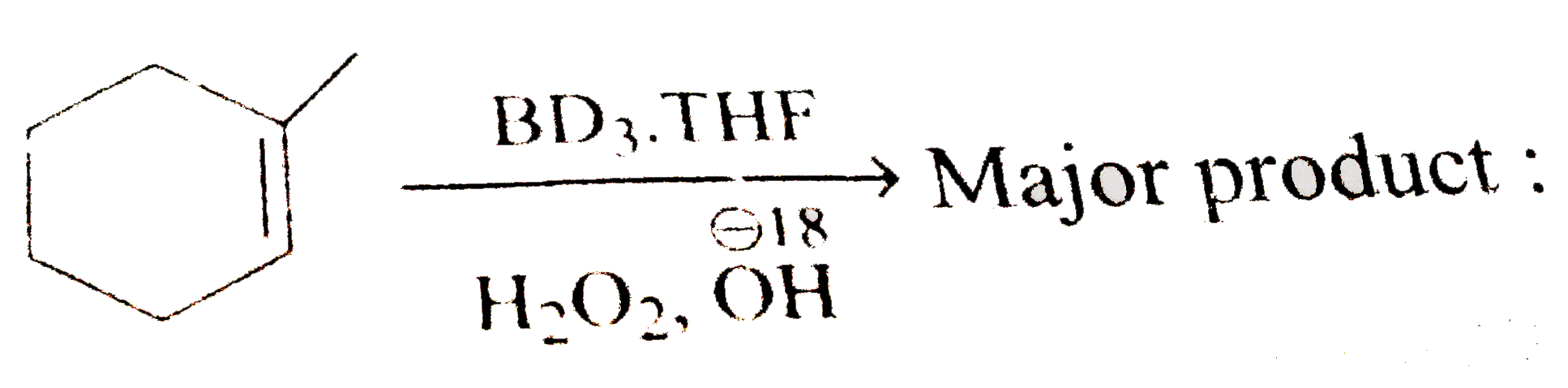A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HYDROCARBONS (ALKANE, ALKENE and ALKYNE)
HIMANSHU PANDEY|Exercise Linked Comprehension Type (Q.26 To Q.33)|8 VideosHYDROCARBONS (ALKANE, ALKENE and ALKYNE)
HIMANSHU PANDEY|Exercise Match The Column|12 VideosHYDROCARBONS (ALKANE, ALKENE and ALKYNE)
HIMANSHU PANDEY|Exercise More Than One Correct (Q.26 To Q.50)|25 Videos
Similar Questions
Explore conceptually related problems
HIMANSHU PANDEY-HYDROCARBONS (ALKANE, ALKENE and ALKYNE)-Linked Comprehension Type (Q.1 To Q.25)
- Addition of X(2) on alkene is electrophilic additon reaction.Reaction ...
Text Solution
|
- Addition of HX on alkene proceed through the formation of carbocation ...
Text Solution
|
- Addition of HX on alkene proceed through the formation of carbocation ...
Text Solution
|
- Addition of HX on alkene proceed through the formation of carbocation ...
Text Solution
|
- Alkene and alkyne both undergo electrophilic additon beacuise of pi e...
Text Solution
|
- Alkene and alkyne both undergo electrophilic additon beacuise of pi e...
Text Solution
|
- Alkene and alkyne both undergo electrophilic additon beacuise of pi e...
Text Solution
|
- Find structure of compound A:
Text Solution
|
- Find structure of compound E:
Text Solution
|
- Find structure of compound D:
Text Solution
|
- Which of the following is compund P?
Text Solution
|
- R and s are:
Text Solution
|
- Identify sturcture of compound T:
Text Solution
|
- Hydroboration oxidation reaction is a process of addtition of H(2)O ac...
Text Solution
|
- Hydroboration oxidation reaction is a process of addtition of H(2)O ac...
Text Solution
|
- Hydroboration oxidation reaction is a process of addtition of H(2)O ac...
Text Solution
|
- Alkane may be prepared from alkyl hlide by Wutrtz method where alkyl h...
Text Solution
|
- Alkane may be prepared from alkyl hlide by Wutrtz method where alkyl h...
Text Solution
|
- Alkane may be prepared from alkyl hlide by Wutrtz method where alkyl h...
Text Solution
|
- Hydrocarbon A (C(7)H(12)) was treated with BH(3) .THF, H(2)O(2), NaOH ...
Text Solution
|