A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMIC STRUCTURE
MOTION|Exercise EXERCISE-2 (LEVEL-II)|31 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-3|30 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-1|77 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 4 | Level-II|16 VideosBIOMOLECULES & POLYMERS
MOTION|Exercise EXERCISE - 4 (LEVEL II)|17 Videos
Similar Questions
Explore conceptually related problems
MOTION-ATOMIC STRUCTURE -EXERCISE-2 (LEVEL-I)
- Assuming Heisenberg Uncertainity Principle to be true what could be th...
Text Solution
|
- An electron is moving with the velocity equal to 10% of the velocity o...
Text Solution
|
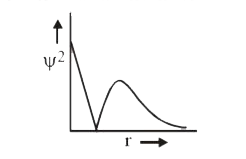
- The following graph between Psi^(2) probability density and distance f...
Text Solution
|
- Which of the following is correct radial probability distribution curv...
Text Solution
|
- Which of the following graphs correspond to one node ?
Text Solution
|
- Suggest the angular and spherical nodes in a.4p b.3p c. 3s
Text Solution
|
- Increasing order of magnetic monent among the following species is ...
Text Solution
|
- Which electronic level would allow the hydrogen atom to absorb a phot...
Text Solution
|
- The orbital angular momentum of an electron in 2s orbital is ………
Text Solution
|
- Which quantum number is not related with Schrodinger equation :-
Text Solution
|
- In the absence of external magnetic field, d-orbital is
Text Solution
|
- In which of the following pairs is the probability of finding the elec...
Text Solution
|
- The maximum probability of finding electron in the d(xy) orbital is -
Text Solution
|
- The number of unpaired electrons in carbon atom is -
Text Solution
|
- When 4 d orbital is complete, the newly entering electrons goes in to ...
Text Solution
|
- Which of the following elements is represented by the electronic confi...
Text Solution
|
- The number of d-electrons in Fe^(2+) (Z= 26 ) is not equal to that o...
Text Solution
|
- Magnetic moment of X^(+3) ion of 3d series is sqrt(35)B.M What is the ...
Text Solution
|
- Which element is represented by the following electronic configuration...
Text Solution
|
- What are the values of the orbital angular momentum of an electron in ...
Text Solution
|