A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
TS EAMCET PREVIOUS YEAR PAPERS-TS EAMCET 2018 (7 MAY SHIFT 1)-CHEMISTRY
- Which of the following statements are correct with respect to benzene ...
Text Solution
|
- A compound having elements X and Y crystallises in a cubic structure, ...
Text Solution
|
- If the degree of association is 70% for the reaction 2A hArr (A) (2) t...
Text Solution
|
- 0.1 mole of NaCl is dissolved in 100g of water. The mole fraction of N...
Text Solution
|
- What is the approximate standard free energy change per mole of Zn (in...
Text Solution
|
- Which of the following graphs represent a first order reaction (a=init...
Text Solution
|
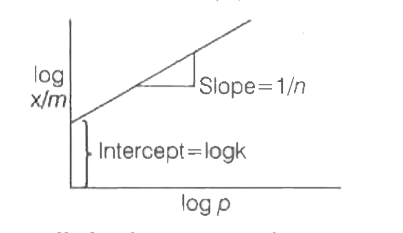
- In the Freundlich isotherm ((x)/(m)=k (p) ^(1//n)) plot of log ""x/m v...
Text Solution
|
- Which of the following element is extracted using I (2) as the reactan...
Text Solution
|
- The equatin and axial P-Cl bond length (in pm) respectively in PCl (5)...
Text Solution
|
- In reactin (1), XeF(6) hydrolysis to form HF and X. In reaction (2), X...
Text Solution
|
- Ethylendiamine (en)
Text Solution
|
- Which one of the followng is square planar in structure and has diamag...
Text Solution
|
- Examples for natural polymers are
Text Solution
|
- Which one of the following statements is correct?
Text Solution
|
- The drug tetracycline is
Text Solution
|
- Which of the following statements is correct for optically active alky...
Text Solution
|
- Which one of the following reactins gives phenol as a major product ?
Text Solution
|
- The products A and B of the below reaction sequence are H-C=C -CH(2)...
Text Solution
|
- The strongest acid among the following is
Text Solution
|
- The order of basicity among the following nitrogen compounds is
Text Solution
|