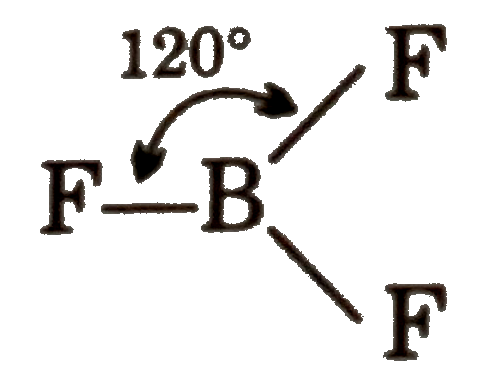A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise comprehension type3|2 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise comprehension type4|1 VideosQUANTUM NUMBERS AND GENERAL CHEMISTRY
GRB PUBLICATION|Exercise comprehension type|5 VideosPRACTICAL ORGANIC CHEMISTRY
GRB PUBLICATION|Exercise Exercise 4 (Matrix match type )|1 VideosREDOX REACTIONS
GRB PUBLICATION|Exercise All Questions|332 Videos
Similar Questions
Explore conceptually related problems