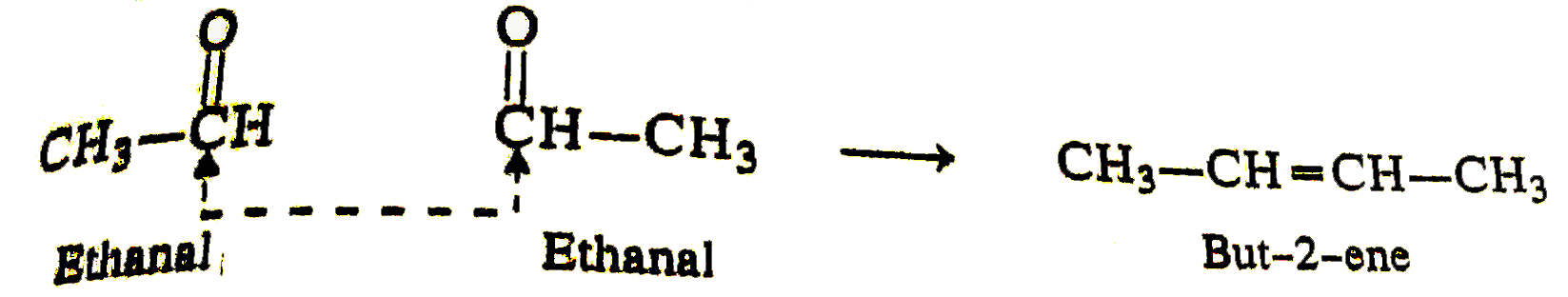Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ALCOHOLS AND PHENOLS-HIGHER ORDER THINKING SKILLS
- An alcohol of unknown structure gave a positive Lucas test in about fi...
Text Solution
|
- Predict the product when alcohol undergoes dehydration with conc. H(2...
Text Solution
|
- Convert CH(3)OH into CH(3)CH(2)OH, CH(3)CH(OH)CH(3) and (CH(3))(3)COH....
Text Solution
|
- Give the structure of the major organic products obtained from 3-ethyl...
Text Solution
|
- 0.037 g of an alcohol, ROH was added to CH(3)MgI and the gas evolved m...
Text Solution
|
- Predict the product of the following reaction :
Text Solution
|
- Explain the formation of A and B when X reacts with C(2)H(5)MgBr.
Text Solution
|
- Complete the following sequence of reactions. Give a suitable mechanis...
Text Solution
|