A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
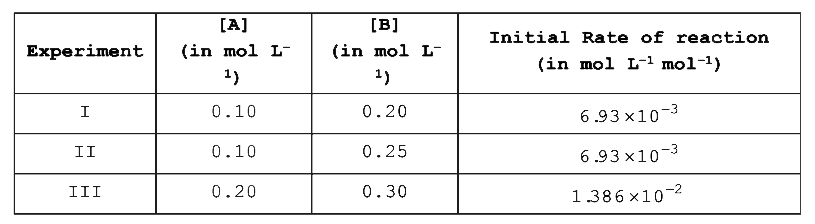
- The following results were obtained during kinetic studies of the reac...
Text Solution
|
- During the kinetic study of the reaction, 2A + B to C + D. Following r...
Text Solution
|
- The following results were obtained during kinetic studies of the reac...
Text Solution
|
- During the kinetic study of the reaction, 2A+BtoC+D , following result...
Text Solution
|
- 2A + B rarr C + D अभिक्रिया की बलगतिकी अध्ययन करने पर निम्नलिखित परिणा...
Text Solution
|
- The following results were obtained during kinetic studies of the reac...
Text Solution
|
- The following results were obtained during kinetic studies of the reac...
Text Solution
|
- During the kinetic study of the reaction, 2A+Brarr C+D , following res...
Text Solution
|
- 2A+B toC+Dअतः अभिक्रिया की बगलतिकी अध्यन करने पर निम्नलिखित परिणाम प्...
Text Solution
|