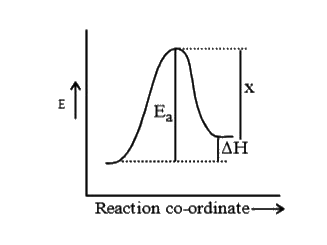
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Chemical Kinetics
MOTION|Exercise SOLVED EXAMPLE (SUBJECTIVE)|24 VideosChemical Kinetics
MOTION|Exercise Exercise -1 (Introduction, Rate of reaction, Factor affecting rate of reaction, Effect of concentration on reaction rate)|9 VideosChemical Kinetics
MOTION|Exercise Exercise - 4 (Level - II) (SUBJECTIVE PROBLEM)|1 VideosCHEMICAL EQUILIBRIUM
MOTION|Exercise Exercise - 4|20 VideosCLASSROOM PROBLEMS
MOTION|Exercise Electrochemistry|22 Videos
Similar Questions
Explore conceptually related problems
MOTION-Chemical Kinetics-SOLVED EXAMPLE (OBJECTIVE)
- In gaseous reactions important for understanding of the upper atmosphe...
Text Solution
|
- In a certain reaction, 10% of the reactant decomposes in one hour, 20%...
Text Solution
|
- Two substances A (t(1//2)=5 min) and B(t(t//2)=10min) are taken in suc...
Text Solution
|
- The reaction A(g)+2B(g)rarrC(g)+D(g) is an elementary process. In an e...
Text Solution
|
- For a hypothetical reaction A+B rarr C+D, the rate =k[A]^(-) ""^(1//2)...
Text Solution
|
- An organic compound A decomposes by following two parallel first order...
Text Solution
|
- For any I^(st) order gaseous reaction A rarr 2B Pressure devoloped af...
Text Solution
|
- For any reaction, 2A rarr B, rate constant of reaction is 0.231" min...
Text Solution
|
- For any reaction A(g) rarr B(g), rate constant k = 8.21 xx 10^(-2) atm...
Text Solution
|
- 2A rarr B+C The mechanism of the above reaction given as 2A overse...
Text Solution
|