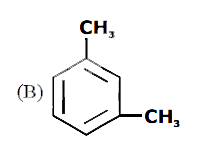
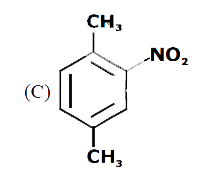
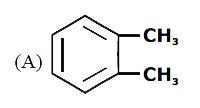A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
AROMATIC COMPOUND
MOTION|Exercise Exercise - 2 (Level-II)|29 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 3|17 VideosAROMATIC COMPOUND
MOTION|Exercise Exercise - 4 | Level-II|16 VideosALKYI HALIDE
MOTION|Exercise Exercise - 4 | Level-II|8 VideosATOMIC STRUCTURE
MOTION|Exercise EXERCISE-4 (LEVEL-II)|18 Videos
Similar Questions
Explore conceptually related problems
MOTION-AROMATIC COMPOUND-Exercise - 2 (Level-I)
- Which of the following is most reactive towards sulphonation ?
Text Solution
|
- When sulphonilic acid (p-H(2)NC(6)H(4)SO(3)H) is treated with excess o...
Text Solution
|
- Ring nitration of dimethyl benzene results in the formation of only on...
Text Solution
|
- If p-methoxy toluene is nitrated, the major product is :
Text Solution
|
- For the electrophilic substitution reaction involving nitration, which...
Text Solution
|
- Identify the correct order of reactivity in electro- philic substituti...
Text Solution
|
- The orbital picture of a singlet carbene (CH(2)) can be drawn as
Text Solution
|
- The orbital picture of a triplet carbene can be drawn as
Text Solution
|
- It is believed that chloroform and hydroxide ion react to produce an e...
Text Solution
|
- CHF(2) Br overset(OH^(-))(rarr) (A) [Intermediate] overset("Trans -2-b...
Text Solution
|
- Ph-underset(14)overset(O)overset("||")(C)-CHN(2) underset(H(2)O)overse...
Text Solution
|
- Which of the following will not give carbylamine reaction
Text Solution
|
- (A) Product (A) will be
Text Solution
|
- overset(I)overset("|")(PhCHBr) overset(PhC equiv CPh)underset("EtOK")(...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Intermediate produced during reimer tiemann’s formlylation will be
Text Solution
|
- Which of the following compound doesn’t gives Hoffmann’s carbyl amine ...
Text Solution
|
- CH(3) – NH(2) + CHCl(3) + KOH rarr major product will be :
Text Solution
|
- What is the product of the following reaction ?
Text Solution
|
- It involves conversion of a carboxylic acid amide into an amine with a...
Text Solution
|