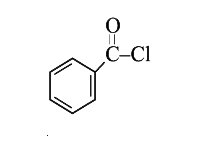
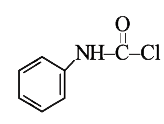
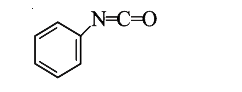
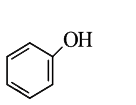A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Aniline reacts with excess of phosgene and KOH to form :
Text Solution
|
- Aniline reacts with excess alkyl halide to give
Text Solution
|
- Aniline reacts with excess of phosgene and KOH to form
Text Solution
|
- Aniline reacts with phosgene to give
Text Solution
|
- Aniline reacts with excess of COCl2 and KOH to form :
Text Solution
|
- Aniline reacts with excess of phosgene and KOH to form :
Text Solution
|
- ऐनिलीन, क्लोरोफॉर्म के साथ KOH की उपस्थिति में अभिक्रिया करके देता है
Text Solution
|
- Aniline reacts with excess of alkyl halide to give
Text Solution
|
- What happens when? (a) Aniline is reacted with acetyl chloride. (b) An...
Text Solution
|