Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
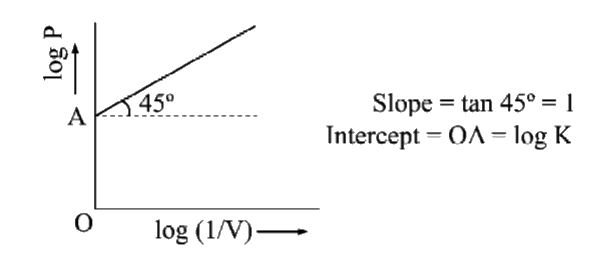
- What is the type of graph between log P and log ((1)/(V)) at constant ...
Text Solution
|
- Draw a graph of log P and log (1//V) for a fixed amount of gas at cons...
Text Solution
|
- Draw a graph of log P vs log (1/V) for a fixed amount of a gas at cons...
Text Solution
|
- The plot of log V against log P at constant temperature for a fixed ma...
Text Solution
|
- (i) The equation of state of a real gas is P(V-b)=RT. Can the gas be l...
Text Solution
|
- What is the slope of the plot of log P vs log V for a given amount of ...
Text Solution
|
- What is the type of graph between log P and log ((1)/(V)) at constant ...
Text Solution
|
- What is the type of graph between log P and log V at constant temperat...
Text Solution
|
- What is the type of graph that would result between log (V) and log ((...
Text Solution
|