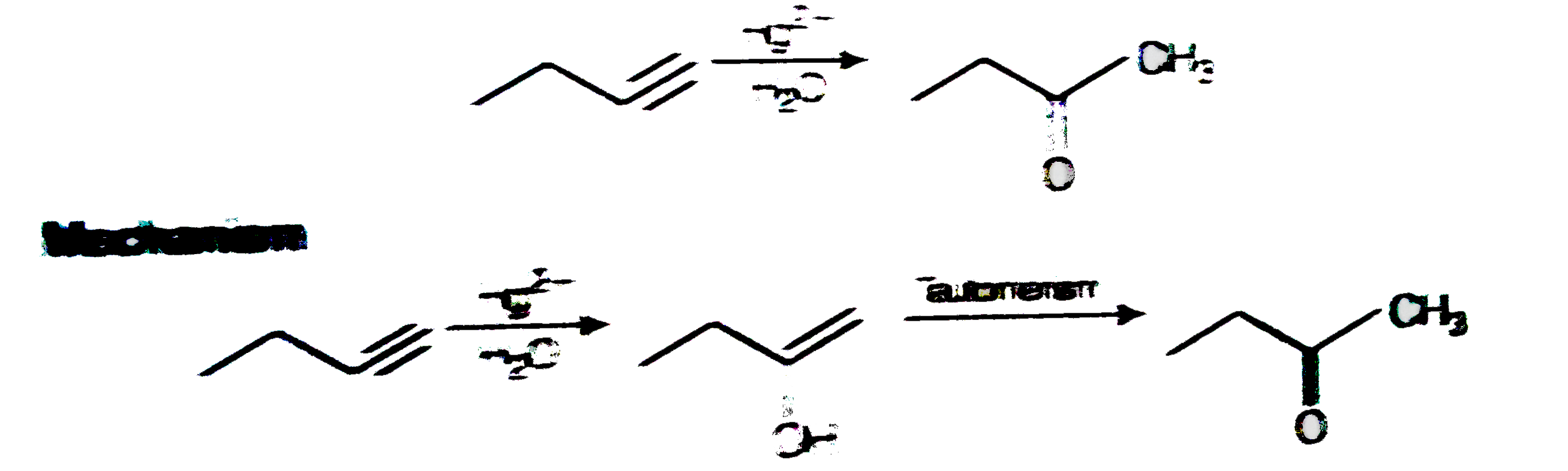
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ALDEHYDE, KETONES AND CARBOXYLIC ACIDS-Aldehyde, Ketones And Carboxylic Acids
- Addition of water to alkyness occurs in acidic medium and in the prese...
Text Solution
|
- Which of the following compounds is the most reactive towards electrop...
Text Solution
|
- The correct order of increasing acidic strength is
Text Solution
|
- Compound Ph-O-overset(O)overset(||)(C)-Ph can be prepared by the react...
Text Solution
|
- The reagent with which both acetaldehyde and acetone react easily is
Text Solution
|
- Cannizzaro's reaction is not given by
Text Solution
|
- Which product is formed when the compound is treated with concentrated...
Text Solution
|
- CH(3) - C -= CHoverset(40%H(2)SO(4))underset(1%HgSO(4))rarrAoverset("i...
Text Solution
|
- Complete the following reaction sequence : CH(3)-overset(O)overset(|...
Text Solution
|
- Which is the most suitable reagent for the following conversion ? CH...
Text Solution
|
- Which of the following compound will give butanone on oxidation with a...
Text Solution
|
- In Clemmensen reduction, carbonyl compound is treated with …….
Text Solution
|
- Which of the following will undergo aldol condensation ?
Text Solution
|
- Treatement of compound Ph-O-overset(O)overset(||)(C)-Ph with NaOH so...
Text Solution
|
- Which of the following conversion can be carried out by Clemmensen red...
Text Solution
|
- Through which of the following reactions number of carbon atoms can be...
Text Solution
|
- Benzophenone can be obtained by ……
Text Solution
|
- Which of the following is the correct represetation for intermediate o...
Text Solution
|
- Why is there a large difference in the boiling points of butanal and b...
Text Solution
|
- Write a test to differentiate between pentan-2-one and pentan-3-one.
Text Solution
|