A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CAREER POINT-MOCK TEST 3-CHEMISTRY
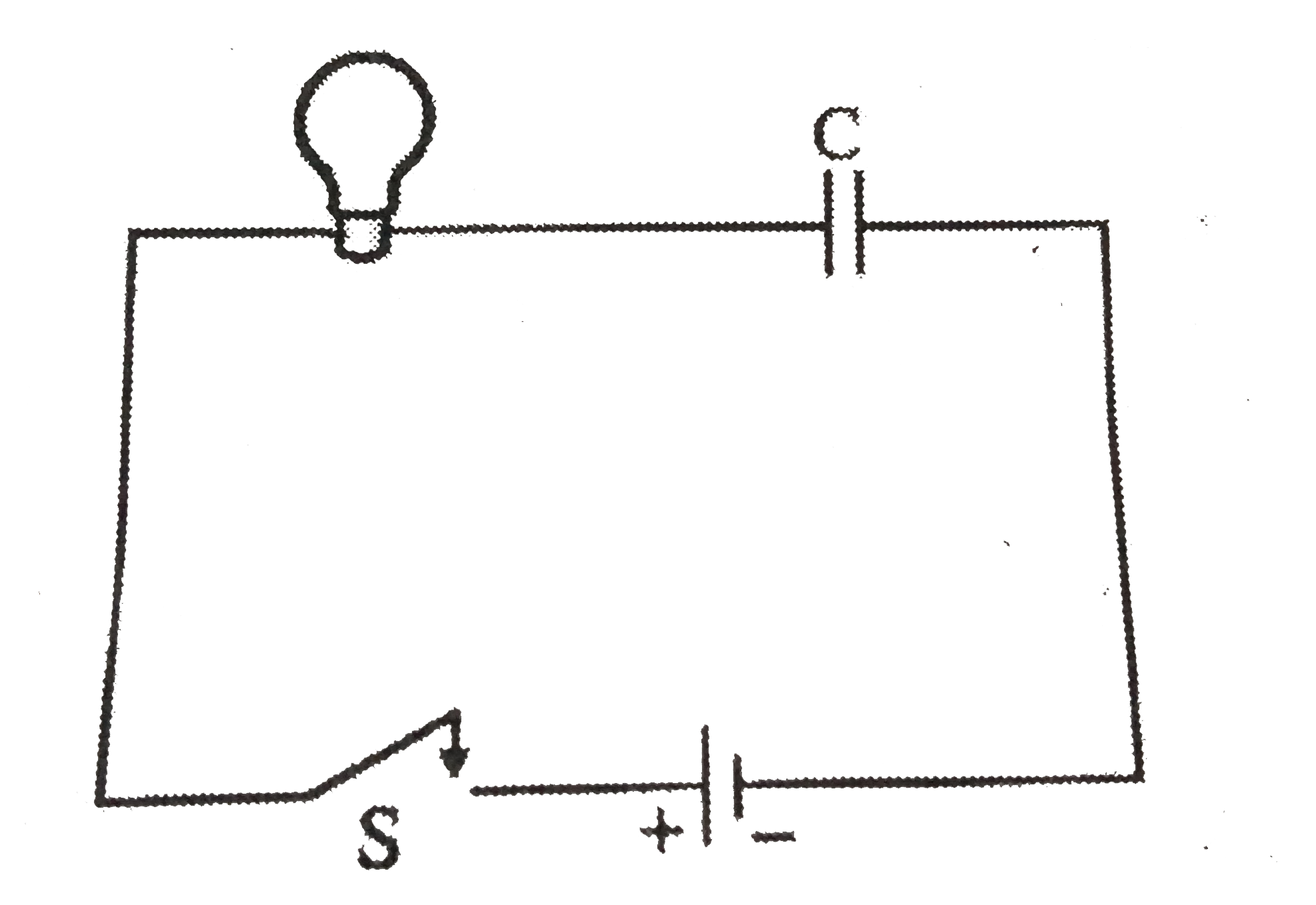
- A light bulb a capacitor and a battery are connected together as shown...
Text Solution
|
- The hybridization states of the nitrogen atom in pyridine, piperdine a...
Text Solution
|
- A sample of Ammonium phosphate (NH(4))(3)PO(4) contains 3.18 moles of ...
Text Solution
|
- Which of the folowing staements is not correct?
Text Solution
|
- Polarisation is the distortion of the shape of the shape of an anion b...
Text Solution
|
- The de-Brogile wavelength of a neutron at 927^(@)C is lamda. What will...
Text Solution
|
- In a closed flask of 5 litre, 1.0 g of H(2) is heated from 300-600K. W...
Text Solution
|
- Assume that the decomposition of HNO(3) can be represented by the foll...
Text Solution
|
- Which of the following is a buffer solution ?
Text Solution
|
- A gaseous system undergo a change of state from (1) to (2) by any of t...
Text Solution
|
- The empty space between the shaded baals and holow balls and hollow ba...
Text Solution
|
- What will be the temperature at which a solution containing 6 g of glu...
Text Solution
|
- In a Cu-voltameter, mass deposited in 30 s is m gm. If the time-curren...
Text Solution
|
- Plotting a graph of log t(1//2) against log [A](0) of a rectant for a ...
Text Solution
|
- The rate of disappearence of SO(2) in the reaction 2SO(2)+O(2)to2SO(...
Text Solution
|
- Which of the following compound have 1^(@),2^(@), 3^(@) and 4^(@)C pre...
Text Solution
|
- Relate the following compounds
Text Solution
|
- Which has maximum b,p and m,p out of :
Text Solution
|
- Action of heat on a mixture of sodium propionate and sodalime produces...
Text Solution
|
- In which case racemisation takes place ?
Text Solution
|
- Which of the following molecule do not give Br(2)/Water test
Text Solution
|