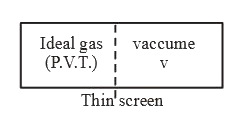Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Thermodynamics
MOTION|Exercise Solved Example|10 VideosThermodynamics
MOTION|Exercise EXERCISE - 1|55 VideosThermodynamics
MOTION|Exercise EXERCISE - 3 SECTION b|26 VideosSURFACE TENSION & VISCOSITY
MOTION|Exercise Exercise - 4 | Level-II Previous Year | JEE Advanced|12 VideosUNIT & DIMENSIONS
MOTION|Exercise Exercise - 4 (Level - II) (PREVIOUS YEAR JEE ADVANCED)|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-QUESTIONS FOR PRACTICE
- If ideal gas expand against vaccum, insulated container then what will...
Text Solution
|
- If a carnot engine works as temperature between 300 to 400 K. then fin...
Text Solution
|
- Efficiency of carnot engine working between ice point and steam point ...
Text Solution
|
- The coeffcient of performance of a refrigerator working between 10^(@)...
Text Solution
|
- An ideal Carnot's engine whose efficiency 40% receives heat of 500K. I...
Text Solution
|
- Intial temperature of a gas is 300 K & its gamma = 1.5 if adibatically...
Text Solution
|
- For monoatomic gas, its initial temperature 127° C if adiabatically it...
Text Solution
|