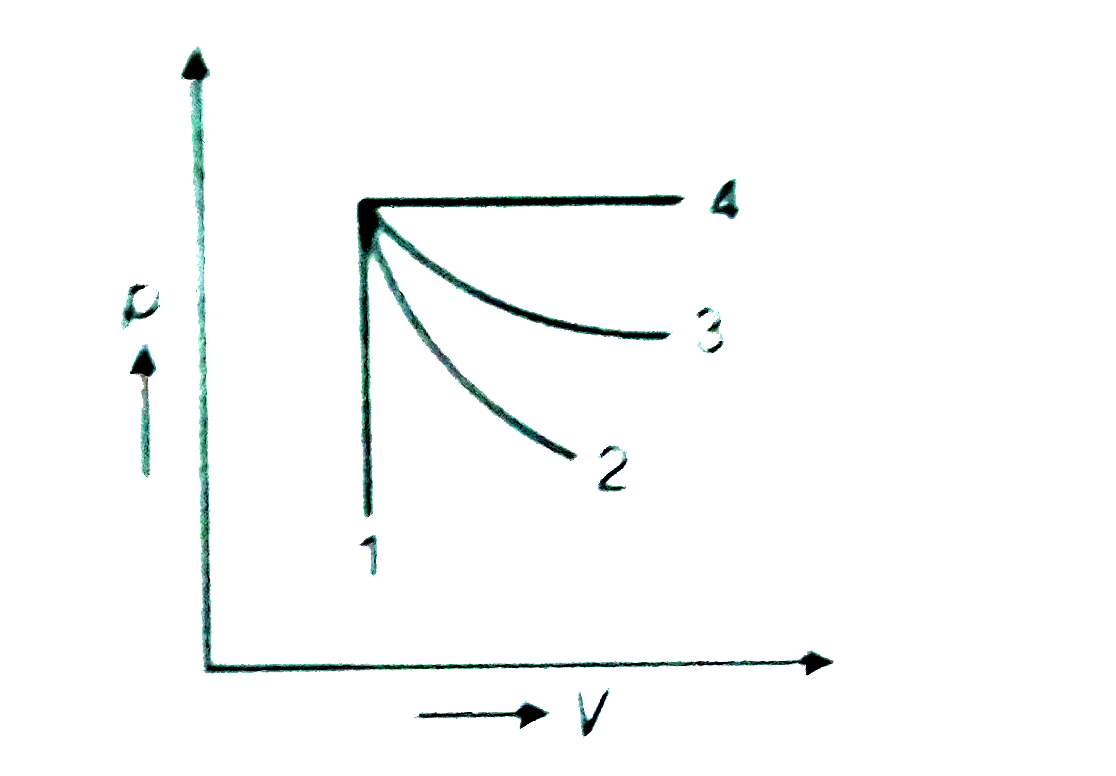
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-THERMODYNAMICS -MULTIPLE CHOICE QUESTIONS (MCQs)
- An ideal gas undergoes for different processes from the same initial s...
Text Solution
|
- An ideal gas undergoes cyclic process ACBC as shown is given PV diagra...
Text Solution
|
- Consider two containers A and B containing identical gases at the same...
Text Solution
|
- Which of the process described below are irrevesible?
Text Solution
|
- An ideal gas undergoes isothermal process from some initial state i to...
Text Solution
|
- (figure). Shows the P-V diagram of an ideal gas undergoing a change of...
Text Solution
|
- A monatomic gas at a pressure P, having a volume V expands isothermall...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- At 27^@C two moles of an ideal monatomic gas occupy a volume V. The ga...
Text Solution
|
- In the above question, change in internal energy of gas is
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in the fi...
Text Solution
|
- The work of 146 kJ is performed in order to compress one kilo mole of ...
Text Solution
|
- In V-T diagram shown in fig., what is the relation between P1 and P2 ?
Text Solution
|
- The P-V diagram of a gas undergoing a cyclic process ABCDA is shown in...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- A body at a temperature of 727^(@)C and having surface area 5 cm^(2), ...
Text Solution
|
- In a Carnot engine when T(2) = 0^(@)C and T(1) = 200^(@)C its efficien...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- The door of a running refrigerator inside a room is left open. The cor...
Text Solution
|
- If a piece of metal is heated to temperature theta and the allowed to ...
Text Solution
|