Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Value Based Questions
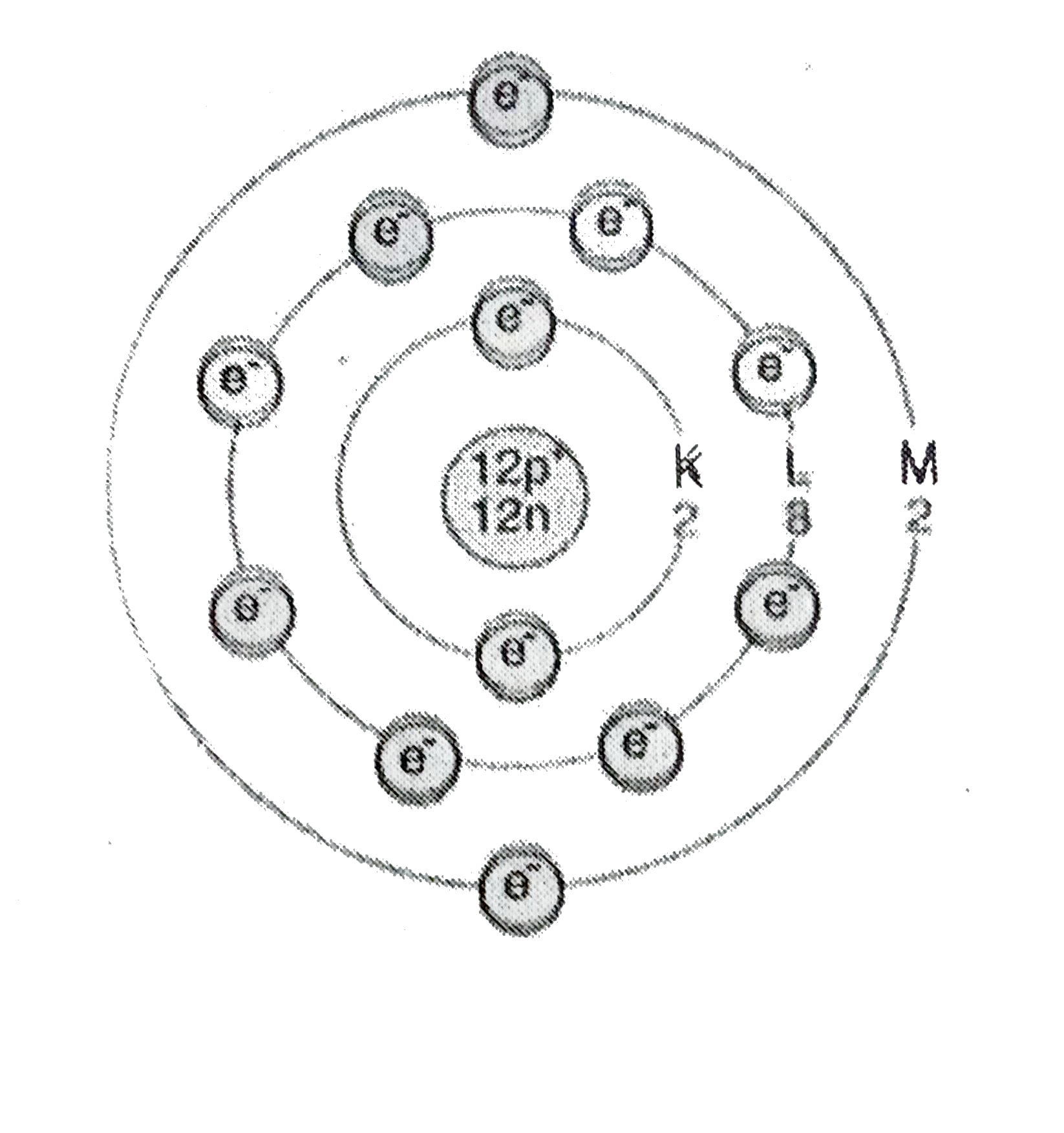
- Write the electronic configuration of an element X whose atomic number...
Text Solution
|
- Ajay and Rakesh live in Rajgarh town of Churu districk of Rajasthan. A...
Text Solution
|
- Roshni is a student of class IX in a school in Delhi. One day Roshni's...
Text Solution
|
- Vibha is a student of 9th standard. Vibha's family has an LPG connecti...
Text Solution
|
- (a) (i) Ethyl mercaptan (ii) Strong smell of ethyl mercaptan (b) D...
Text Solution
|
- Abhinav is a student of class IX. One day all the students were perfor...
Text Solution
|
- All the students of class 9 were performing experiments to study the t...
Text Solution
|
- Raghvan is a student of class 9 in a Chennai school. His teacher, Mr. ...
Text Solution
|
- Rohit and Arun are two friends both of whom study in class IX in diffe...
Text Solution
|
- Bhavna is a student of class IX in a city school. She has a five year ...
Text Solution
|
- Bunny is a ten year old boy. His motherm Mrs. Bhatia, is having high f...
Text Solution
|
- Pawan is a student of class IX. The students of his class have recentl...
Text Solution
|
- One day Vabhav was performing experiments in the science laboratory ba...
Text Solution
|
- Pratap is a student of class IX in a city school. He has recently stud...
Text Solution
|
- Vikram is a student of class IX in a village school. He lives on the o...
Text Solution
|
- Ramnik is a student of class 9. One day he was studying a chapter of c...
Text Solution
|
- Naveen is a student of class IX in a city school. His uncle Ram Dev wh...
Text Solution
|
- Akshay and Sauranh are the students of class IX. They have recently st...
Text Solution
|