Text Solution
Verified by Experts
Topper's Solved these Questions
STRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Very Short Answer|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise NCERT Book, Page 50|1 VideosSTRUCTURE OF ATOM
LAKHMIR SINGH & MANJIT KAUR|Exercise Value Based Questions|17 VideosMODEL TEST PAPER 5
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|2 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-STRUCTURE OF ATOM-Exercise
- On the basis of Thomson’s model of an atom, explain how the atom is ne...
Text Solution
|
- On the basis of Rutherford’s model of an atom, which sub- atomic parti...
Text Solution
|
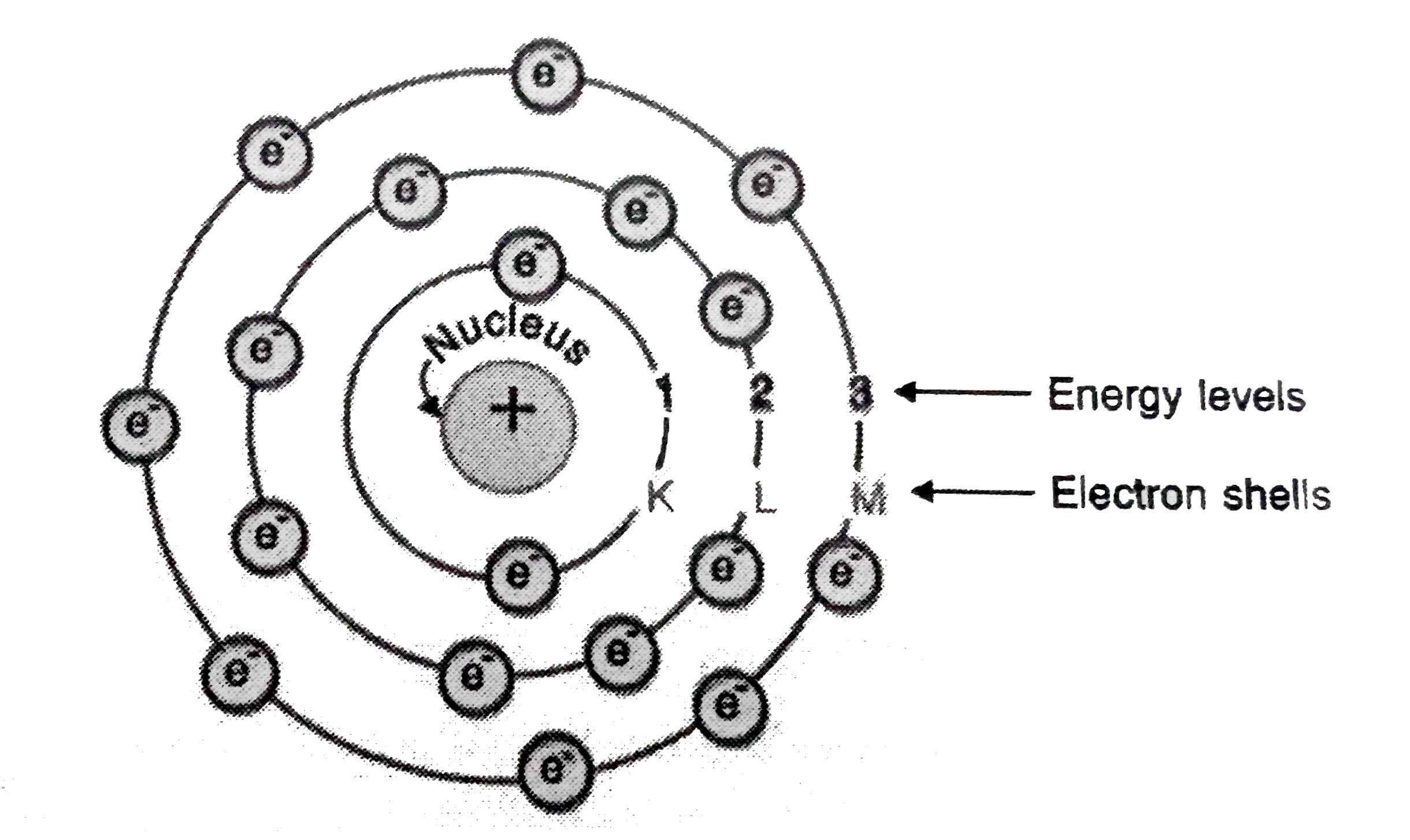
- Draw a sketch of Bohr’s model of an atom with three shells.
Text Solution
|
- What do you think would be the observation if the alpha-particle scatt...
Text Solution
|
- Name the three sub-atomic particles of an atom.
Text Solution
|
- Helium atom has an atomic mass of 4u and two protons in its nucleus. H...
Text Solution
|
- If K and L shells of an atom are full, then what would be the total nu...
Text Solution
|
- How will you find the valency of chlorine, sulphur and magnesium ?
Text Solution
|
- If number of electrons in an atom is 8 and number of protons is also 8...
Text Solution
|
- With the help of Table given below, find out the mass numbers of oxyge...
Text Solution
|
- For the symbol H,D and T tabulate three sub-atomic particles found in ...
Text Solution
|
- Write the electronic configuration of any one pair of isotopes and iso...
Text Solution
|
- Compare the properties of electrons, protons and neutrons.
Text Solution
|
- What are the limitations of J.J. Thomson’s model of the atom?
Text Solution
|
- What are the limitations of Rutherford's model of the atom ?
Text Solution
|
- Describe Bohr's model of the atom.
Text Solution
|
- Compare all the proposed models of an atom given in this chapter.
Text Solution
|
- Summarise the rules for writing of distribution of electrons in variou...
Text Solution
|
- Define valency by taking examples of silicon and oxygen.
Text Solution
|
- Explain with examples (i) Atomic number (ii) Mass number (iii) Isotope...
Text Solution
|