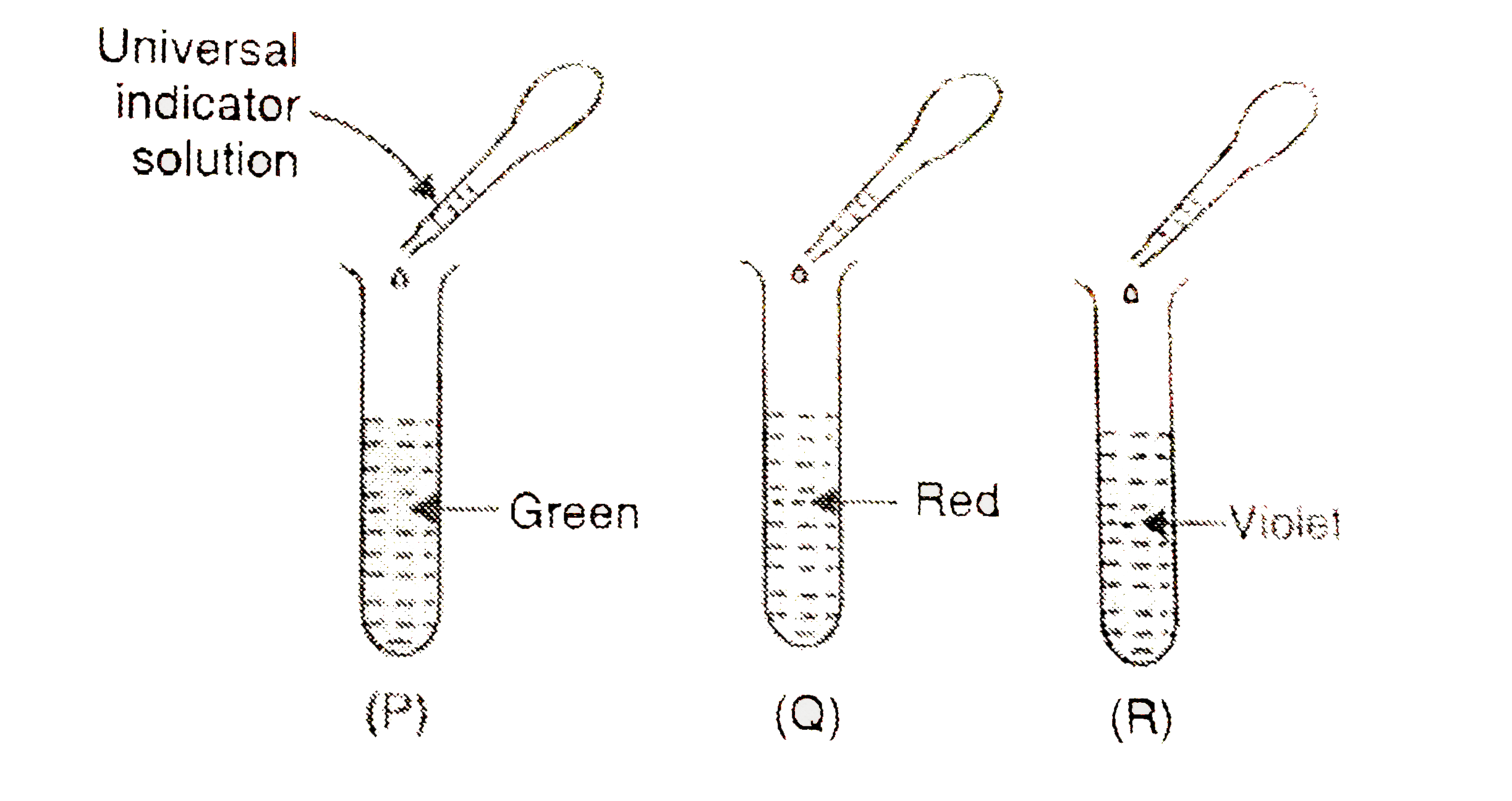A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise SHORT ANSWER TYPE|8 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise LONG ANSWER TYPE|3 VideosPERIODIC CLASSIFICATION OF ELEMENTS
LAKHMIR SINGH & MANJIT KAUR|Exercise VERY SHORT ANSWER TYPE|6 VideosMETALS AND NON-METALS
LAKHMIR SINGH & MANJIT KAUR|Exercise Short Answer Type Qustions|1 VideosTEST PAPER 1
LAKHMIR SINGH & MANJIT KAUR|Exercise Section B|4 Videos
Similar Questions
Explore conceptually related problems
LAKHMIR SINGH & MANJIT KAUR-PERIODIC CLASSIFICATION OF ELEMENTS-Exercise
- 2mL of ethanoic acid was taken in each of test tube I and test tube II...
Text Solution
|
- Four students A,B,C and D were asked by their teacher to arrange the s...
Text Solution
|
- On adding a few drops of universal indicator solution to three unknown...
Text Solution
|
- A few drops of ethanoic acid were added to solid sodium carbonate. The...
Text Solution
|
- Did Döbereiner’s triads also exist in the columns of Newlands’ Octaves...
Text Solution
|
- What were the limitations of Döbereiner’s classification?
Text Solution
|
- What were the limitations of Newlands’ Law of Octaves?
Text Solution
|
- Use Mendeléev’s Periodic Table to predict the formulae for the oxides ...
Text Solution
|
- Besides gallium, which other elements have since been discovered that ...
Text Solution
|
- What were the criteria used by Mendeléev in creating his Periodic Tabl...
Text Solution
|
- Why do you think the noble gases are placed in a separate group?
Text Solution
|
- How could the Modern Periodic Table remove various anomalies of Mendel...
Text Solution
|
- Name two elements you would expect to show chemical reactions similar ...
Text Solution
|
- Name (a) three elements that have a single electron in their outermo...
Text Solution
|
- (a) Lithium, sodium, potassium are all metals that react with water to...
Text Solution
|
- In the Modern Periodic Table, which are the metals among the first ten...
Text Solution
|
- By considering their position in the Periodic Table, which one of the ...
Text Solution
|
- Which of the following statements is not a correct statement about the...
Text Solution
|
- Element X forms a chloride with the formula XCl(2), which is a solid w...
Text Solution
|
- Which element has (a) two shells, both of which are completely filled...
Text Solution
|