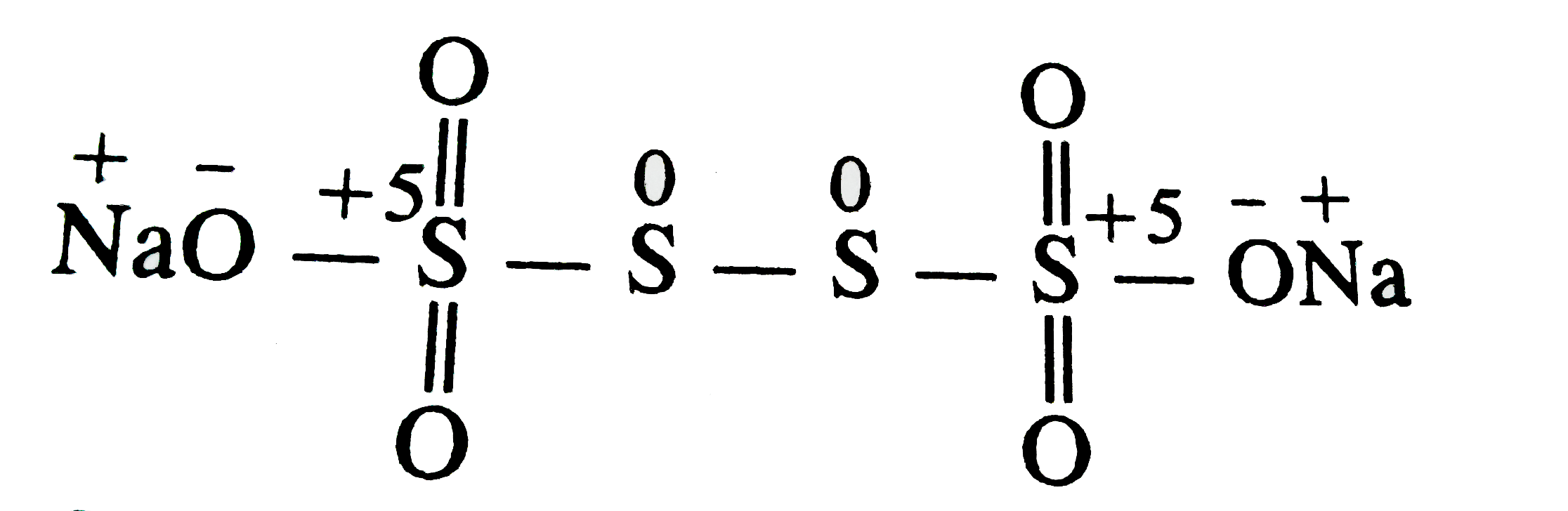
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE OXYGEN FAMILY
DINESH PUBLICATION|Exercise REASON ASSERTION|17 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise PREPARATORY PACKAGE|14 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise MATRIX MATCH|2 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 VideosTHE P-BLOCK ELEMENTS
DINESH PUBLICATION|Exercise Brain Storming Multiple Choice Questions (McQs)|9 Videos
Similar Questions
Explore conceptually related problems