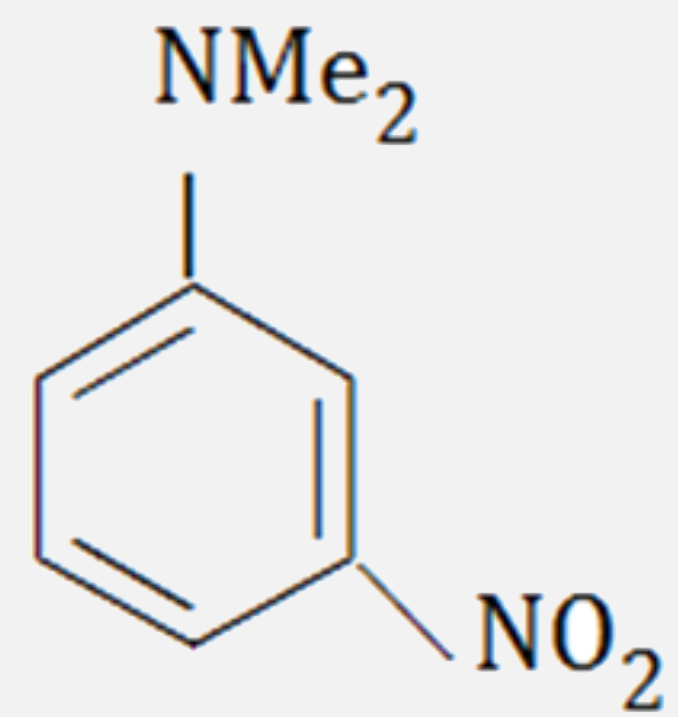
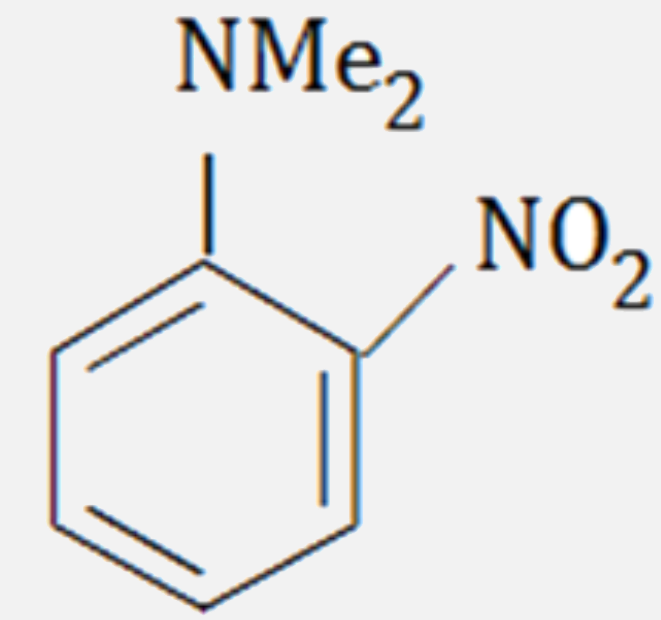
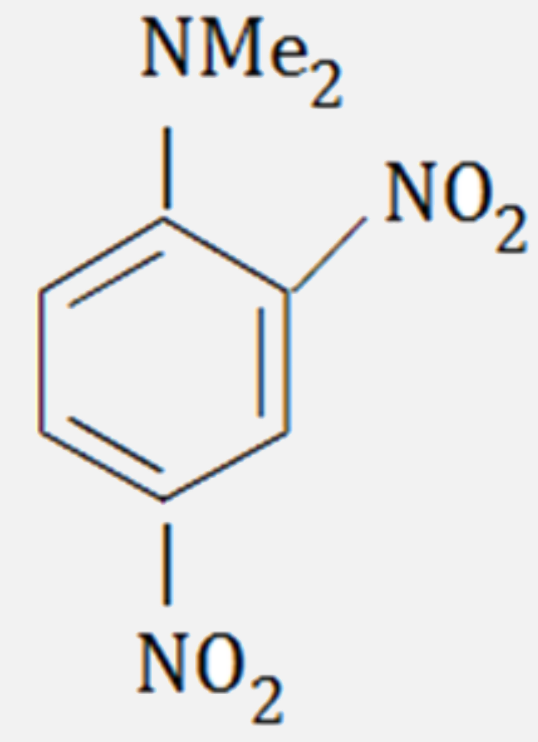
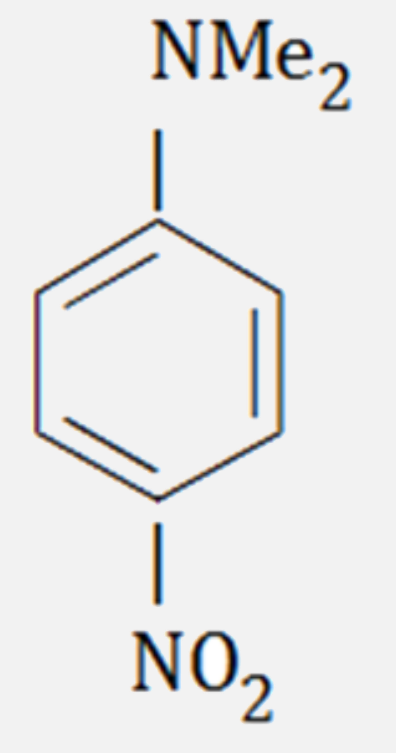
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 79-CHEMISTRY
- 0.85% aqueous solution of NaNO(3) is apparently 90% dissociated at 27^...
Text Solution
|
- What is spectrochemical series?
Text Solution
|
- The major product formed on nitration of N, N - dimethylaniline with c...
Text Solution
|
- How many grams of a dibasic acid (Mol. Wt. =200) should be present in ...
Text Solution
|
- Which of the following compounds would be most ionic in character ?
Text Solution
|
- Consider the following reaction at equilibrium 2Fe^(2+)(aq)+Cu^(2+)to2...
Text Solution
|
- Find the incorrect statement
Text Solution
|
- The numbers of radial nodes of 3s and 2p orbitals are respectively:
Text Solution
|
- The hyperconjugative stabilities of tert-butyl cation and 2-butene, re...
Text Solution
|
- The crystals of ferrous sulphate on heating give
Text Solution
|
- 2,4 - DNP is obtained by reacting hydrazine hydrate with which of the ...
Text Solution
|
- On heating with dilute sulphuric acid, naphthalene - 1 sulphonic acid ...
Text Solution
|
- Consider the following reduction processes : Zn^(2+)+2e^(-)toZn(s), ...
Text Solution
|
- The following compounds have been arranged in order of their increasin...
Text Solution
|
- Among the carboxylic acid shown below, the ones that exhibit stereoiso...
Text Solution
|
- Which of the following statements is correct about heat of combustion?
Text Solution
|
- An organic compound undergoes first decompoistion. The time taken for ...
Text Solution
|
- Aniline can be distinguished from methyl amine by its reaction with
Text Solution
|
- C2 - epimer of D - Glucose is
Text Solution
|
- Which of the following alkene in acid catalysed hydration form 2-methy...
Text Solution
|