A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 98-CHEMISTRY
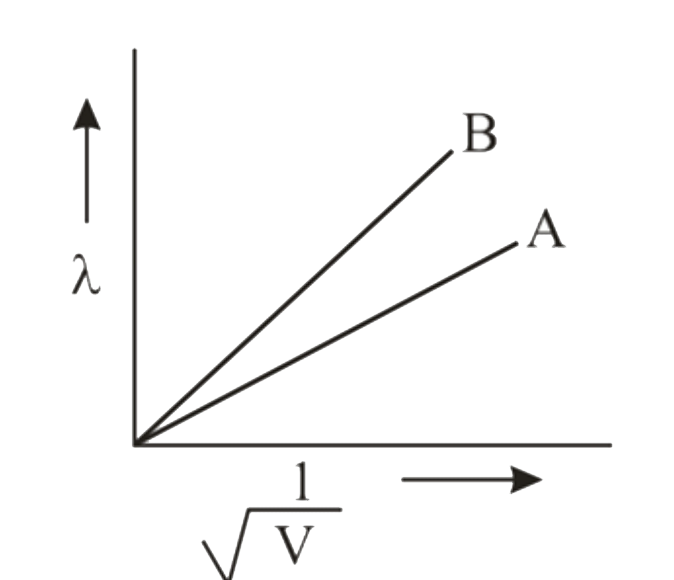
- de Broglie wavelengths of two particles A and B are plotted against (1...
Text Solution
|
- Which of the following compounds would you expect to have a dipole mom...
Text Solution
|
- In which of the following processes colloidal sulphur is formed
Text Solution
|
- Which of the following weights the least ?
Text Solution
|
- Which of the following will have highest bond energy ?
Text Solution
|
- Which one of the following ion is aromatic ?
Text Solution
|
- A gas cylinder containing cooking gas can withsand a pressure of 14.9 ...
Text Solution
|
- For the reaction CH3COOH(l)+C2H5(l)hArrCH3COOC2H5(l)+H2O(l) the value ...
Text Solution
|
- The IUPAC name of the following compound is
Text Solution
|
- Atoms of elements B from hep lattice and those of element A occupy two...
Text Solution
|
- Number of structural isomers of C4H(10)O that are ethers are
Text Solution
|
- In the given reaction CH3-underset(C3H7)underset(|)overset(C2H5)overse...
Text Solution
|
- Which of the following statement is not applicable to H2SO4 ?
Text Solution
|
- The half life period for catalytic decomposition of XY3 at 100 mm is f...
Text Solution
|
- The sodium salt of a weak acid is hydrolysed to the extent of 3% in 0....
Text Solution
|
- Consider the following compounds (1) C6H5-NH-CH3 (2) C6H5-NH-C6H5 (3) ...
Text Solution
|
- The concentration aqueous solution of potassium salts of acetic acid a...
Text Solution
|
- Which of the following carbonyl compounds will give recemisation react...
Text Solution
|
- Which transition metal has lowest density ?
Text Solution
|
- A certain quantity of electricity deposits 0.54 g of Ag from silver ni...
Text Solution
|