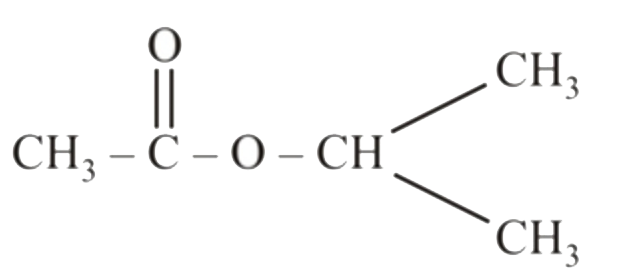
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 98-CHEMISTRY
- How will you convert butan -2-one to propanoic acid ?
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (l) and benz...
Text Solution
|
- poling process is used for
Text Solution
|
- The vapour pressure of benzene at 90^@C is 1020 torr. A solution of 15...
Text Solution
|
- What is the % dissociation of H(2)S if 1 "mole" of H(2)S is introduced...
Text Solution
|
- Prussic acid +O2overset("Ag")rarr The products of above reaction are
Text Solution
|
- Consider the following statements 1. Cs^+ ion is more highly hydrate...
Text Solution
|
- The root mean square velocity of an ideal gas in a closed container of...
Text Solution
|
- A gaseous reaction X2(g)rarrZ(g)+1/2Y(g) shows increase in pressure fr...
Text Solution
|
- Which one of the following nitroalkanes will give nitrolic acid with N...
Text Solution
|
- Unknown compound A C9H8O5 on methylation with (CH3)SO4//NaOH forms met...
Text Solution
|
- Consider the M(OH)(3) formed by all the group 13 elements. The correct...
Text Solution
|
- In the reaction
Text Solution
|
- Which one of the following can shown optical isomerism ?
Text Solution
|
- Pick out incorrect statement.
Text Solution
|
- Which of the following compounds liberates dihydrogen gas at anode whe...
Text Solution
|
- The standard reduction potential for Cu^(2+)//Cu is +0.34V. Calculate ...
Text Solution
|
- Sodium salt of acetic acid reacts with ethyl iodide to give
Text Solution
|
- The C-Cl bond length is shortest in :
Text Solution
|
- In the given reaction (X) will be
Text Solution
|