A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NEET PREVIOUS YEAR (YEARWISE + CHAPTERWISE)-THERMODYNAMICS-QUESTIONS
- Given the bond energies of H - H and Cl - Cl are 430 kJ mol^(-1) and 2...
Text Solution
|
- Consider the following reactions: (i) H^(+)(aq)+OH^(-)(aq)rarrH(2)O(...
Text Solution
|
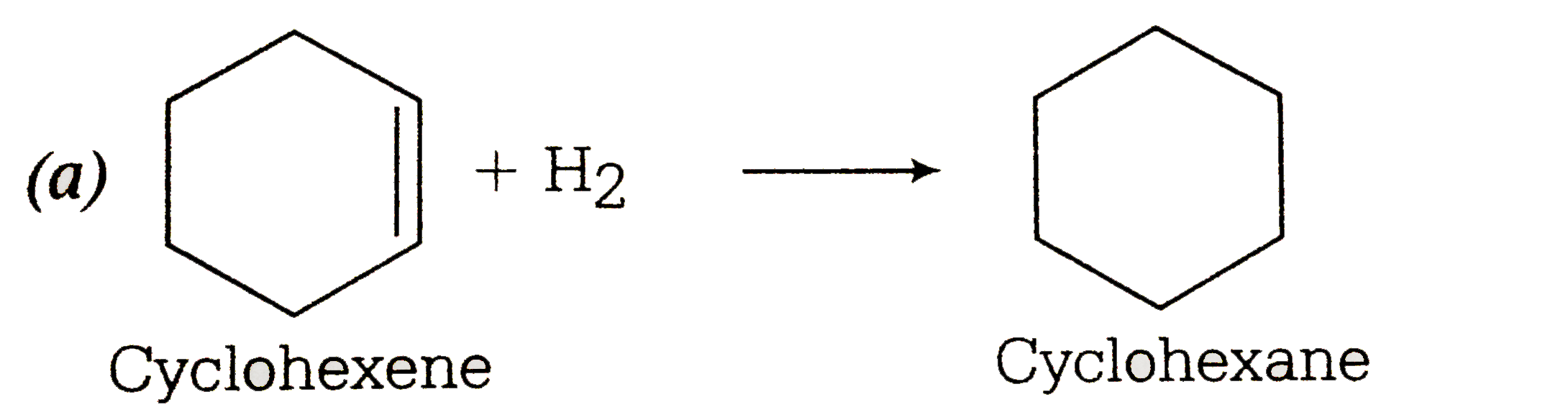
- The enthalpy of combustion of H(2) , cyclohexene (C(6)H(10)) and cyclo...
Text Solution
|
- The enthalpy and entropy change for the reaction, Br(2)(l)+Cl(2)(g)r...
Text Solution
|
- Assume each reaction is carried out in an open container. For which ...
Text Solution
|
- Identify the correct statement for change of Gibbs energy for a system...
Text Solution
|
- The absolute enthalpy of neutralisation of the reaction MgO(s) + 2HC...
Text Solution
|
- Which of the following pairs of a chemical reaction is certain to resu...
Text Solution
|
- A reaction occurs spontanecously if :-
Text Solution
|
- The work done during the expanision of a gas from a volume of 4 dm^(3)...
Text Solution
|
- Considering entropy (S) as a thermodynamics parameter, the criterion f...
Text Solution
|
- Standard enthalpy and standard entropy change for the oxidation of NH(...
Text Solution
|
- The bond energies of H--H , Br--Br and H--Br are 433,, 192 and 364KJmo...
Text Solution
|
- For the reaction C(3)H(8)(g)+5O(2)rarr3CO(3)(g)+4H(2)O(l) at const...
Text Solution
|
- For which one of the following equation is DeltaH(reaction)^(@) equal ...
Text Solution
|
- What is the entropy change (in JK^(-1)mol^(-1)) when one mole of ice i...
Text Solution
|
- The molar heat capacity of water at constant pressure, C, is 75 JK^(-1...
Text Solution
|
- The densities of graphite and diamond at 298K are 2.25 and 3.31gcm^(-3...
Text Solution
|
- Heat of combustion DeltaH^(@) for C(s),H(2)(g) and CH(4)(g) are 94, -6...
Text Solution
|
- 2 mol of an ideal gas at 27^(@)C temperature is expanded reversibly fr...
Text Solution
|