A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
HALOALKANES AND HALOARENES
OP TANDON|Exercise Single Interger answer type questions|14 VideosHALOALKANES AND HALOARENES
OP TANDON|Exercise Matrix -Match Type Questions|8 VideosELECTROCHEMISTRY
OP TANDON|Exercise Matrix-Matching Type Question|3 VideosISOMERISM (STRUCTURAL AND STEROISOMERISM)
OP TANDON|Exercise INTEGER|7 Videos
OP TANDON-HALOALKANES AND HALOARENES -Linked comprehension type questions passage
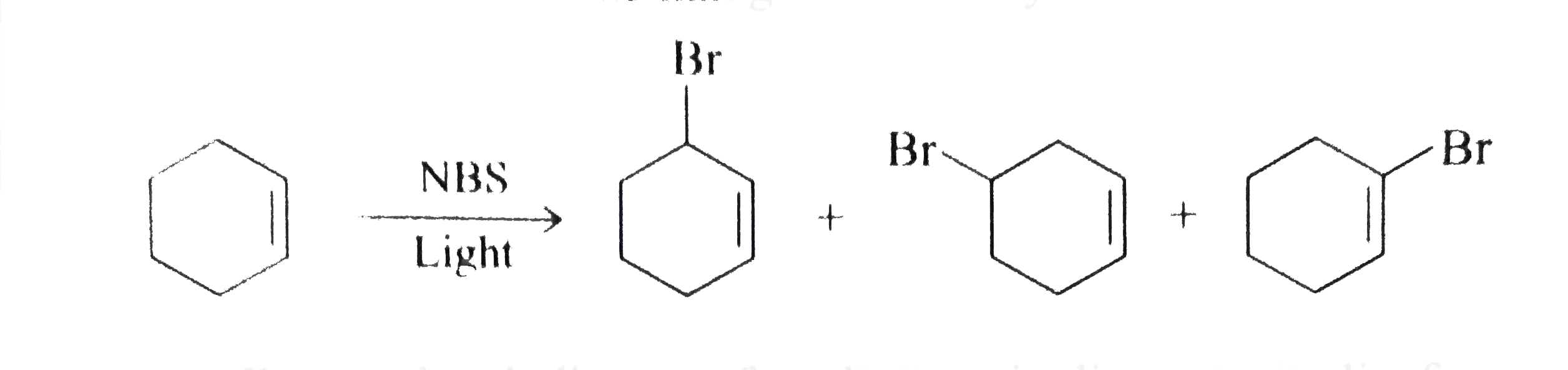
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- Karl Ziegler reported that alkenes react with N-bromosuccinimide (NBS)...
Text Solution
|
- The removal of two atoms or groups one generally hydrogen (H^(+)) and ...
Text Solution
|
- The removal of two atoms or groups one generally hydrogen (H^(+)) and ...
Text Solution
|
- The removal of two atoms or groups one generally hydrogen (H^(+)) and ...
Text Solution
|
- The removal of two atoms or groups one generally hydrogen (H^(+)) and ...
Text Solution
|
- The removal of two atoms or groups one generally hydrogen (H^(+)) and ...
Text Solution
|
- S(N^(1)) reaction is a first order nucleophilic substitution e.g. CH...
Text Solution
|
- S(N^(1)) reaction is a first order nucleophilic substitution e.g. CH...
Text Solution
|
- S(N^(1)) reaction is a first order nucleophilic substitution e.g. CH...
Text Solution
|
- S(N^(1)) reaction is a first order nucleophilic substitution e.g. CH...
Text Solution
|
- S(N^(1)) reaction is a first order nucleophilic substitution e.g. CH...
Text Solution
|
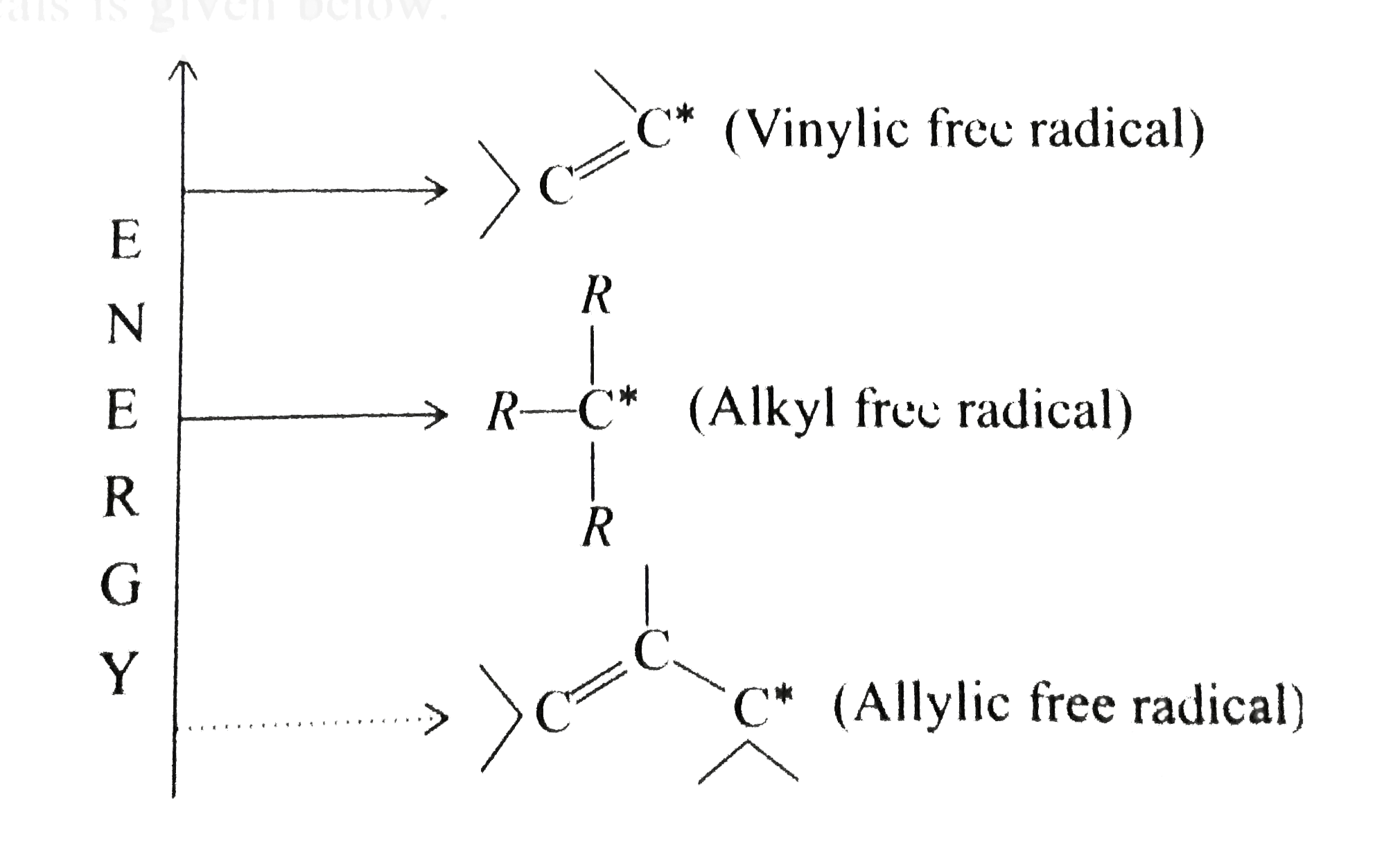
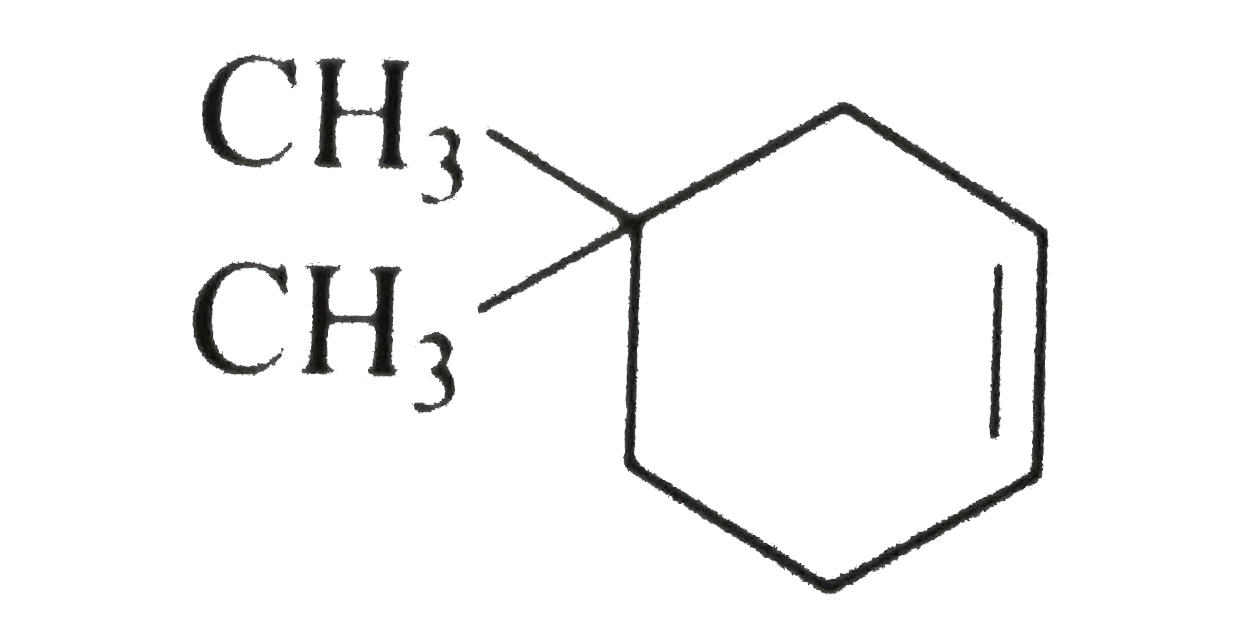
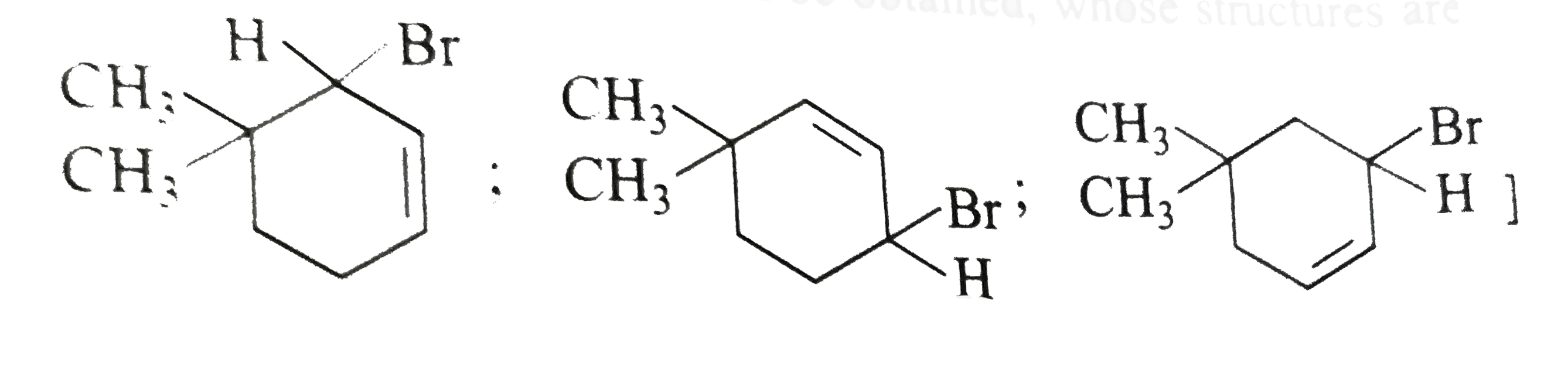
- Free radical halogenation takes place in the presence of light or at h...
Text Solution
|
- Free radical halogenation takes place in the presence of light or at h...
Text Solution
|
- Free radical halogenation takes place in the presence of light or at h...
Text Solution
|
- Free radical halogenation takes place in the presence of light or at h...
Text Solution
|
- Free radical halogenation takes place in the presence of light or at h...
Text Solution
|
- For a typical nucleophilic aromatic substitution reaction to take plac...
Text Solution
|
- For a typical nucleophilic aromatic substitution reaction to take plac...
Text Solution
|