A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 104-CHEMISTRY
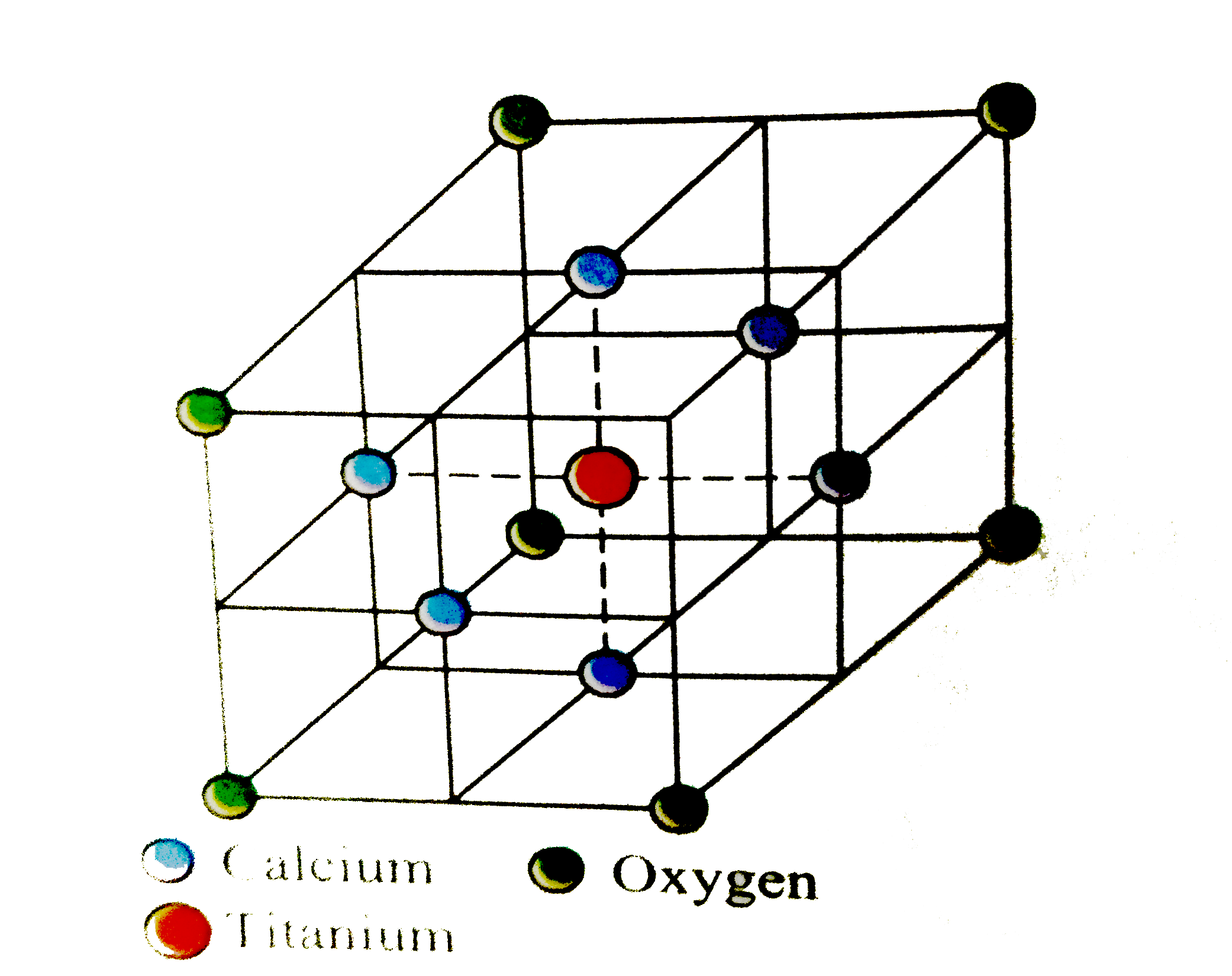
- Perovaskite, a mineral containing calcium, oxygen & titanium crystall...
Text Solution
|
- Least stable hydride is
Text Solution
|
- Which of the following combination does not liberated NH(3) gas?
Text Solution
|
- 14.2 g Na2SO4 is present in 10^2 kg water. Its concentration in ppm is
Text Solution
|
- Metallic magnesium is prepared by
Text Solution
|
- Arrange basicity of given compounds in decreasing order
Text Solution
|
- The ratio a/b (the terms used in van der Waals' equation) has the unit...
Text Solution
|
- In the reaction, CH3 - CH2-CBr3overset("Ag Powder"//Delta)(rarr)(X)....
Text Solution
|
- SO2+NO+H2O to X+ a dibasic acid X overset(NaNH2)(rarr)Y + H2O In the...
Text Solution
|
- The de-Broglie wavelength of a neutron at 1327^@C is lambda . What wil...
Text Solution
|
- Which of the following molecules involves the formation of dpi - p pi ...
Text Solution
|
- When ethyl iodide and n-propyl iodide are allowed to react with sodium...
Text Solution
|
- Which of the following halides cannot be hydrolysed at room temperatur...
Text Solution
|
- The equilibrium constants for the reaction Br(2)hArr 2Br at 500 K an...
Text Solution
|
- Glycol forms diethylene glycol on heating with
Text Solution
|
- Oxidation number if iodine in IO(3)^(-), IO(4)^(-),KI and I(2) respect...
Text Solution
|
- Which of the following complex will give white precipitate with barium...
Text Solution
|
- In the given reaction C(6)H(5)-OHoverset(H2SO4)(rarr) underset("as m...
Text Solution
|
- The enthalpy change for a reaction does not depend upon:
Text Solution
|
- How long a current of 3 ampere has to be passed through a solution of ...
Text Solution
|