Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-ACIDS, BASES AND SALTS -LAQs LONG ANSWER QUESTIONS
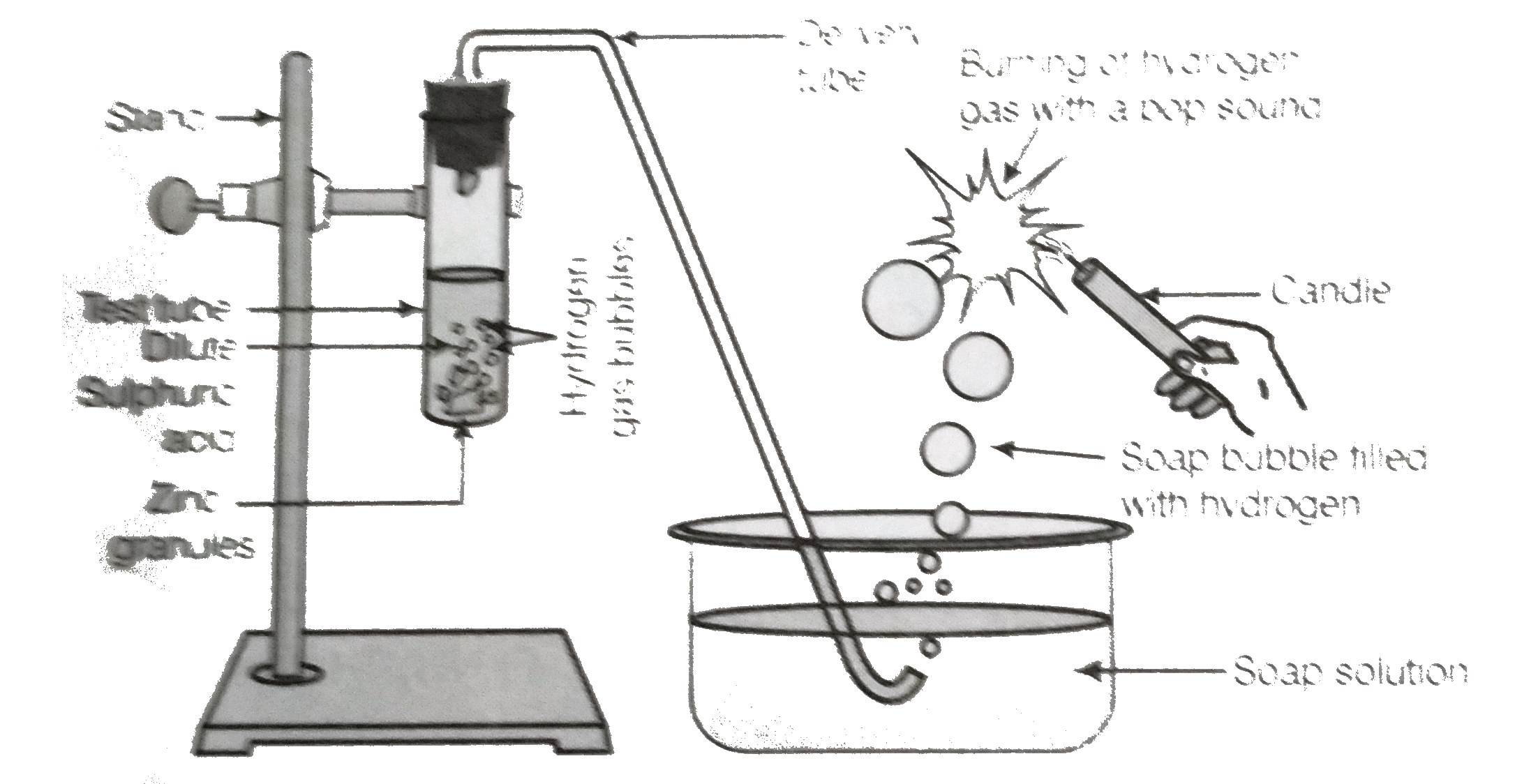
- In the following schematic diagram form the preapratinn of hydrogen ga...
Text Solution
|
- For making cake, baking p[owder is taken . If at home your mother uses...
Text Solution
|
- A metal cabonate X on rectin g with an acid agives a gas which when p...
Text Solution
|
- A dry pellet of a common base B, When kept in open absorbs moisture an...
Text Solution
|
- A suphlate slat of goup 2 elemne t of the periodic tables is a swhite...
Text Solution
|
- Identify the commpound 'Y' on the basis of the reactin given below. Al...
Text Solution
|